Have you ever wondered how nutrients reach every cell in your body or how plants get water from the soil? These intricate processes are governed by two fundamental principles in biology: diffusion and osmosis. Understanding these concepts is crucial for appreciating the fundamental mechanisms that underpin life as we know it. Whether you’re a student grappling with these concepts for the first time or a seasoned biology enthusiast seeking to solidify your knowledge, this comprehensive guide and answer key will help you navigate the complexities of diffusion and osmosis with ease.
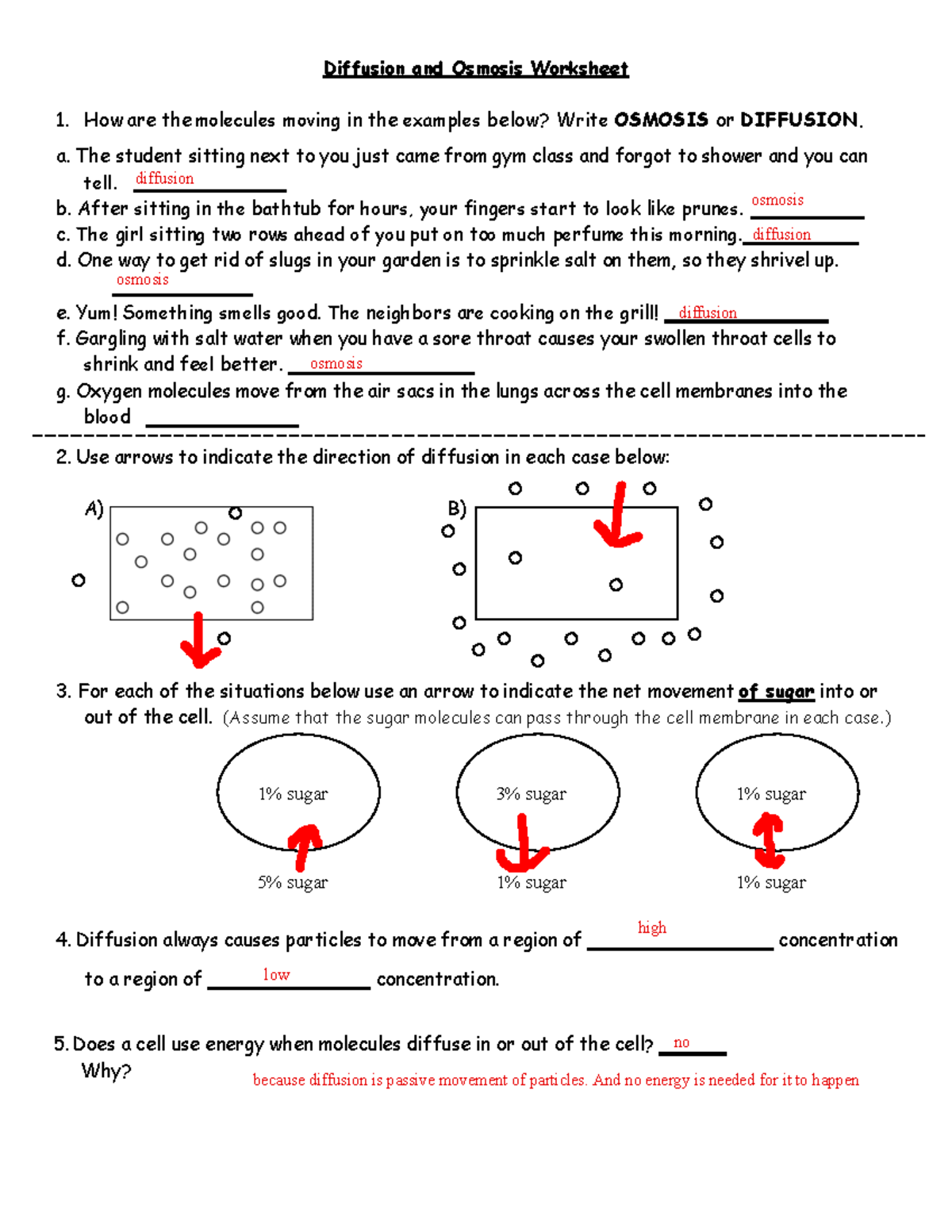
Image: jacoby-well-ellis.blogspot.com
Diffusion and osmosis are often confused, but they have distinct yet interconnected roles in living systems. Diffusion, the simpler of the two, describes the movement of particles from an area of higher concentration to an area of lower concentration. Imagine a drop of ink spreading through a glass of water. The ink molecules, initially concentrated in one spot, gradually disperse throughout the water until they are evenly distributed. Osmosis, on the other hand, focuses on the movement of water across a semipermeable membrane, a barrier that allows certain molecules to pass through but blocks others. The driving force behind osmosis is the difference in water concentration, with water moving from an area of higher water concentration to an area of lower water concentration, until equilibrium is reached.
Delving into Diffusion
Diffusion is a fundamental principle that governs how molecules move and interact within a system. Here’s a breakdown of the key aspects:
Understanding the Driving Force Behind Diffusion
The movement of molecules in diffusion isn’t random. It’s driven by a fundamental principle known as the second law of thermodynamics, which states that systems tend towards a state of greater disorder or randomness. This means that molecules naturally tend to spread out and distribute themselves evenly, leading to a more stable and less ordered state.
Factors Influencing Diffusion Rate
The rate at which diffusion occurs is influenced by several factors:
- Concentration Gradient: The steeper the concentration gradient, meaning the greater the difference in concentration between two areas, the faster the rate of diffusion. Think of a perfume bottle: the scent diffuses more rapidly in a still room than it does in a breezy one.
- Temperature: Higher temperatures increase the kinetic energy of molecules, causing them to move faster and thus diffuse more rapidly. In a hot room, the scent of perfume will spread faster than in a cold room.
- Surface Area: A larger surface area allows for more contact points between the diffusing substance and the surrounding medium, leading to faster diffusion. This is why lungs have a large surface area for efficient gas exchange.
- Molecular Size: Smaller molecules diffuse faster than larger ones because they can navigate through the medium more easily. Imagine a small ball rolling faster than a larger one in the same scenario.
- Distance: The shorter the distance between the two areas, the faster the rate of diffusion. This is why cells need to be small to ensure efficient diffusion of nutrients and waste products.

Image: db-excel.com
Real-World Examples of Diffusion
Diffusion plays a vital role in various biological and everyday processes:
- Cellular Respiration: In cells, oxygen diffuses into the mitochondria, where it’s used for cellular respiration to produce energy, while carbon dioxide, a waste product, diffuses out of the cell. This continuous exchange of gases is crucial for cellular life.
- Gas Exchange in the Lungs: In the lungs, oxygen diffuses from the alveoli, tiny air sacs, into the bloodstream, and carbon dioxide diffuses from the blood into the alveoli for exhalation. This exchange is essential for our survival.
- Food Coloring in Water: The spreading of food coloring in water is a classic example of diffusion. The dye molecules distribute themselves evenly throughout the water, demonstrating the movement of substances from high to low concentrations.
- Fragrance of Perfume: When you apply perfume, the scent molecules diffuse through the air, spreading the fragrance and eventually allowing others to detect it.
Exploring Osmosis: Water’s Journey Across Membranes
Osmosis, a special case of diffusion, focuses on the movement of water molecules across semipermeable membranes. These membranes, often present in cells, act as barriers, allowing certain molecules to pass through while restricting others. Osmosis is crucial for maintaining cell volume, regulating nutrient transport, and ensuring proper cell function.
The Role of Semipermeable Membranes
Semipermeable membranes play a pivotal role in osmosis. They are selectively permeable, meaning they allow certain substances to pass through while blocking others. This selectivity is based on size, charge, and chemical properties of the molecules. For example, cell membranes are selectively permeable, allowing water molecules to pass through while blocking larger molecules like proteins.
Understanding Osmotic Pressure
Water movement across a semipermeable membrane is driven by a difference in water potential, known as osmotic pressure. The higher the concentration of solutes (dissolved substances) in a solution, the lower its water potential. Water naturally flows from a region of higher water potential (lower solute concentration) to a region of lower water potential (higher solute concentration) to reach equilibrium.
Types of Osmotic Solutions
Depending on the solute concentration inside and outside a cell, the solution can be classified into three types:
- Isotonic: When the solute concentration inside and outside the cell is equal, the solution is called isotonic. Water movement occurs equally in both directions, resulting in no net change in cell volume.
- Hypotonic: If the solute concentration outside the cell is lower than inside, the solution is hypotonic. Water moves into the cell, causing it to swell. This can lead to the bursting of cells, especially in animal cells that lack a rigid cell wall.
- Hypertonic: When the solute concentration outside the cell is higher than inside, the solution is hypertonic. Water moves out of the cell, causing it to shrink. This process is called plasmolysis in plant cells, where the cell membrane detaches from the cell wall due to water loss.
Osmosis in Action: Real-World Examples
- Water Absorption by Plants: Plants absorb water from the soil through their roots using osmosis. The concentration of solutes in the root cells is higher than in the soil water, creating a water potential gradient that drives water into the plant.
- Kidney Function: In the kidneys, osmosis plays a crucial role in filtering waste products from the blood and producing urine. Water moves across the nephron’s semipermeable membranes, regulating the concentration of solutes in various compartments.
- Cell Transport: Osmosis helps maintain cell volume and transports nutrients and waste products across cell membranes. The movement of water across these membranes ensures proper cell function and overall organismal homeostasis.
Diffusion and Osmosis: A Dynamic Duo
Diffusion and osmosis work together to maintain life’s delicate balance. They ensure that substances are transported across cellular and organismal boundaries, allowing organisms to obtain nutrients, eliminate waste, and maintain proper cell function.
Mastering Diffusion and Osmosis Worksheet
Now that you have a thorough understanding of diffusion and osmosis, you’re ready to tackle any worksheet that comes your way! Here’s a breakdown of common questions and how to approach them:
Types of Questions
- Define Diffusion and Osmosis: Be precise and concise in your definitions. Emphasize the concepts of movement of molecules down a concentration gradient for diffusion and water movement across a semipermeable membrane for osmosis.
- Factors Affecting Diffusion: Understand the factors that influence the rate of diffusion, such as concentration gradient, temperature, surface area, molecular size, and distance. Be prepared to explain how these factors affect the speed of movement.
- Osmotic Pressure: Explain the concept of osmotic pressure and how it drives water movement across semipermeable membranes. Relate osmotic pressure to the concentration of solutes in a solution.
- Types of Osmotic Solutions: Define isotonic, hypotonic, and hypertonic solutions, and describe their effects on cells. Be able to draw diagrams illustrating the movement of water in each scenario.
- Real-World Applications: Provide examples of diffusion and osmosis in everyday life and biological systems. Explain how these phenomena are crucial for plant growth, animal physiology, and overall organismal function.
- Problem-Solving: Be prepared to solve numerical problems involving the calculation of diffusion rates, osmotic pressure, or the movement of water across membranes. Use formulas provided in the worksheet or textbooks to arrive at correct answers.
Answer Key for Typical Diffusion and Osmosis Worksheet Questions
Here are some key answers to common questions found on diffusion and osmosis worksheets.
1. What is diffusion?
Diffusion is the net movement of molecules from an area of high concentration to an area of low concentration. This movement is passive, meaning it doesn’t require energy input, and is driven by the second law of thermodynamics.
2. What is osmosis?
Osmosis is a special case of diffusion where water molecules move across a semipermeable membrane from a region of higher water potential (lower solute concentration) to a region of lower water potential (higher solute concentration). This movement is also passive and driven by the difference in water potential.
3. List the factors affecting the rate of diffusion.
- Concentration gradient: The steeper the gradient, the faster the diffusion rate.
- Temperature: Higher temperatures increase the rate of diffusion.
- Surface area: Larger surface area increases the diffusion rate.
- Molecular size: Smaller molecules diffuse faster than larger ones.
- Distance: The shorter the distance, the faster the diffusion rate.
4. Explain how osmotic pressure affects water movement across a membrane.
Osmotic pressure is the pressure that must be applied to a solution to prevent the inward flow of water across a semipermeable membrane. It’s directly proportional to the concentration of solutes in the solution. The higher the solute concentration, the higher the osmotic pressure, leading to a stronger driving force for water movement.
5. Describe the effects of an isotonic, hypotonic, and hypertonic solution on a cell.
- Isotonic: No net movement of water occurs, and the cell maintains its normal volume.
- Hypotonic: Water moves into the cell, causing it to swell. Animal cells may burst, while plant cells maintain their structure due to their cell wall.
- Hypertonic: Water moves out of the cell, causing it to shrink. Animal cells shrivel, while plant cells undergo plasmolysis, detaching their cell membrane from the cell wall.
6. Provide examples of diffusion and osmosis in real-world scenarios.
Diffusion:
- Food coloring spreading in water.
- Oxygen diffusing from the lungs into the bloodstream.
- Fragrance of perfume spreading in the air.
Osmosis:
- Water absorption by plant roots.
- Kidney filtration and urine production.
- Nutrient transport and waste removal across cell membranes.
7. Calculate the rate of diffusion using the provided formula.
To solve this, use the provided formula in the worksheet, plugging in the given variables. For instance, if the formula is: Rate of diffusion = (Concentration gradient x Surface area) / Distance, and the concentration gradient is 10, surface area is 5, and distance is 2, then the rate of diffusion would be (10 x 5) / 2 = 25 units.
Diffusion And Osmosis Worksheet Answer Sheet
Mastering Diffusion and Osmosis: A Journey for Lifelong Learning
Understanding diffusion and osmosis opens a window into the fascinating world of cellular processes and life itself. Diffusion and osmosis are not just abstract concepts; they are the driving forces behind vital processes like respiration, nutrient absorption, and waste removal.
As you embark on your journey of learning about diffusion and osmosis, remember that it is an ongoing process of discovery. There’s always more to learn and explore, especially as new research sheds light on the intricate mechanisms of these fundamental processes. By mastering these concepts, you equip yourself with invaluable tools for understanding the interconnectedness of life and the remarkable efficiency of nature.
Don’t hesitate to delve deeper into this captivating realm. Consult additional resources, engage in discussions with fellow learners, and conduct experiments to solidify your knowledge. The journey of learning about diffusion and osmosis is an enriching one, opening doors to a deeper appreciation of the complexities and wonders of life.






