Remember those long nights hunched over your chemistry textbook, struggling to memorize the periodic table and its intricate electronic configurations? It wasn’t exactly a thrilling experience, right? Well, I’m here to tell you that understanding the periodic table and its electronic configurations doesn’t need to be a chore. With the right resources, like a well-designed periodic table PDF with detailed electronic configurations, you can unlock the secrets of the elements and boost your chemistry understanding.
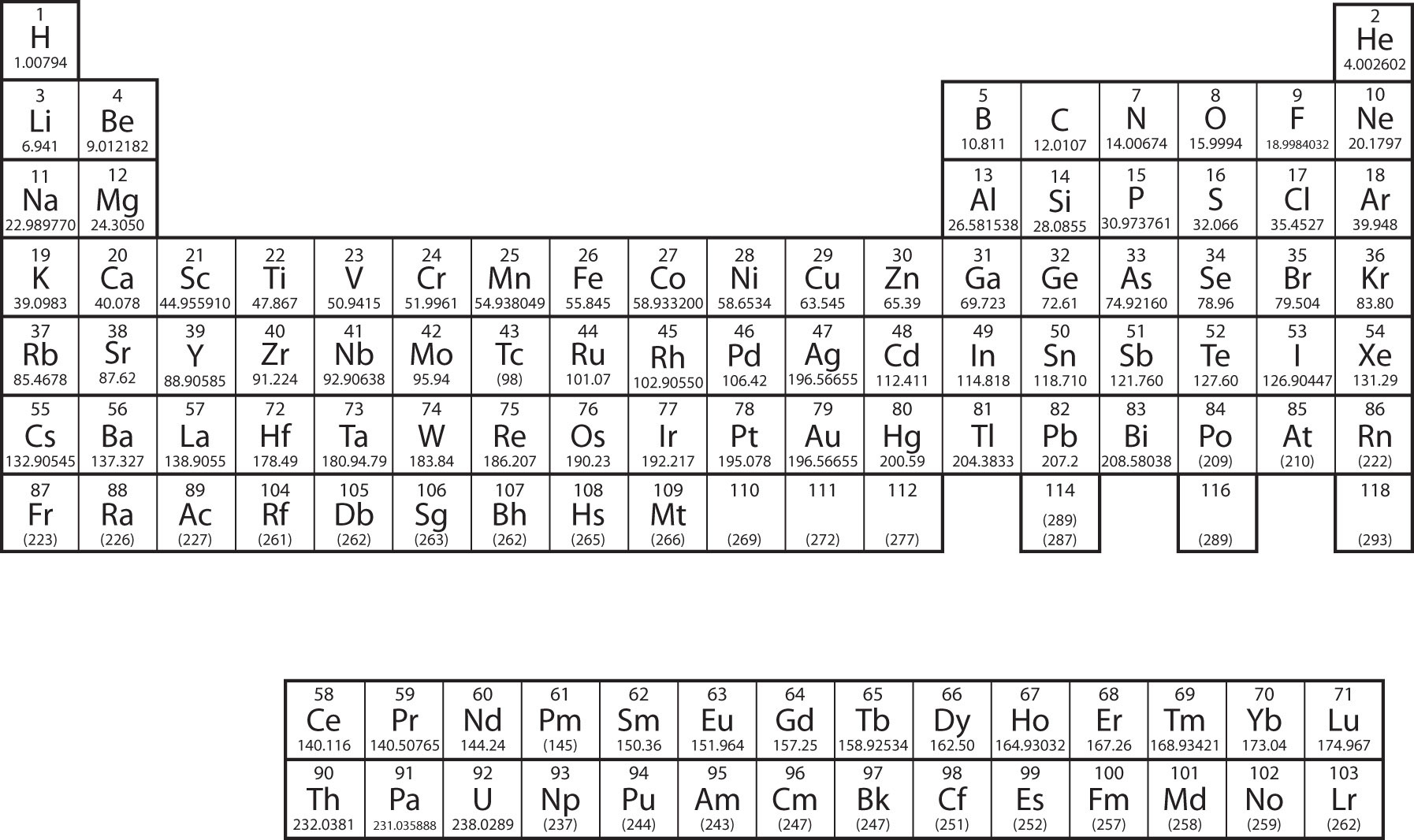
Image: saylordotorg.github.io
This comprehensive guide will delve into the world of the periodic table and its essential electronic configurations. We’ll explore the history, structure, and meaning behind this indispensable tool, while providing you with valuable tips on how to best utilize it for your studies. So, get ready to demystify the periodic table and embrace the power of knowledge!
Understanding the Periodic Table and Electronic Configurations
The periodic table, a cornerstone of chemistry, is a systematic arrangement of the chemical elements in order of increasing atomic number (number of protons in an atom’s nucleus). It is structured into rows (periods) and columns (groups), with elements grouped based on their shared chemical properties and repeating patterns of electron configuration.
Electronic configurations, on the other hand, describe the arrangement of electrons in an atom’s energy levels and sublevels. They provide valuable insights into an element’s reactivity, bonding behavior, and overall chemical properties. By knowing an element’s electronic configuration, you can predict its behavior in chemical reactions and understand its place within the periodic table.
Essential Information on a Periodic Table PDF with Electronic Configurations
A well-designed periodic table PDF with electronic configurations will be your go-to resource for understanding the elements and their properties. It should clearly organize the elements into periods and groups, highlighting each element’s atomic number, symbol, atomic mass, and most importantly, its electronic configuration. Additionally, an ideal PDF will include:
- A clear and concise explanation of the periodic table’s structure and organization.
- A detailed explanation of electronic configurations, including the principles governing electron filling.
- Visual aids like diagrams and charts to illustrate concepts and relationships.
- A section dedicated to understanding the periodic trends, such as electronegativity, ionization energy, and atomic radius.
How to Use a Periodic Table PDF with Electronic Configurations
Once you have your trusted periodic table PDF with electronic configurations, here are some ways to maximize its potential:

Image: sciencenotes.org
1. Start with the Basics:
Begin by understanding the fundamental structure of the table, including the periods and groups. Remember that elements in the same group have similar chemical properties due to their identical number of valence electrons (electrons in the outermost shell).
2. Master Electronic Configurations:
Learn how to determine the electronic configuration of an element by following the rules of filling orbitals. The periodic table itself can act as a guide for writing electronic configurations. For instance, the first two columns (Groups 1 and 2) correspond to the filling of the s orbitals, while the next six columns (Groups 3-8) correspond to the filling of the p orbitals.
3. Explore Periodic Trends:
Utilize the periodic table PDF to investigate the trends in various properties, such as electronegativity, ionization energy, and atomic radius. These trends are directly influenced by the electronic configurations and help predict an element’s reactivity and bonding behavior.
Tips and Expert Advice
Use your periodic table PDF with electronic configurations as a springboard to explore more complex concepts. Consult trusted chemistry textbooks for detailed explanations and practice problems. Additionally, join online forums or social media groups dedicated to chemistry, where you can connect with fellow learners and experienced professionals.
Don’t hesitate to ask questions and seek clarification whenever needed. A solid understanding of the periodic table and electronic configurations is crucial for success in chemistry.
FAQ
Q: What is the role of electronic configuration in predicting an element’s reactivity?
A: An element’s reactivity depends on the number of valence electrons, which are directly related to its electronic configuration. Elements with one or two valence electrons tend to be highly reactive as they easily lose these electrons to achieve a stable configuration. In contrast, elements with a full valence shell (eight electrons) are typically less reactive as they are already stable.
Q: How can a periodic table PDF with electronic configurations be helpful for understanding chemical bonding?
A: Electronic configurations play a crucial role in chemical bonding. When atoms bond, they share or transfer valence electrons to achieve a stable, noble gas electronic configuration. Understanding the electronic configurations of atoms involved in a bond helps predict the type of bond formed (ionic or covalent) and the resulting molecule’s properties.
Q: Are there any online resources for interactive periodic tables with electronic configurations?
A: Yes, there are several online resources offering interactive periodic tables with electronic configurations. These resources allow you to explore the periodic table in a visual and dynamic way, often providing additional information and animation to enhance your learning experience.
Periodic Table Pdf With Electronic Configuration
https://youtube.com/watch?v=S17Glbw3OLc
Conclusion
The periodic table PDF with electronic configurations is a valuable tool for anyone studying chemistry. It provides a comprehensive overview of the chemical elements, enabling a deeper understanding of their properties and behavior. Combine this powerful resource with dedicated study, online resources, and collaboration with other learners, and you’ll be well on your way to mastering the mysteries of the periodic table!
Are you ready to embark on your own journey to explore the fascinating world of the periodic table and electronic configurations?






