Have you ever wondered why a campfire feels warm, but an ice pack makes your skin feel cold? The answer lies in the fascinating world of chemical reactions, specifically endothermic and exothermic reactions. These two types of reactions, while seemingly opposite, govern countless processes around us, from the food we eat to the energy we consume!
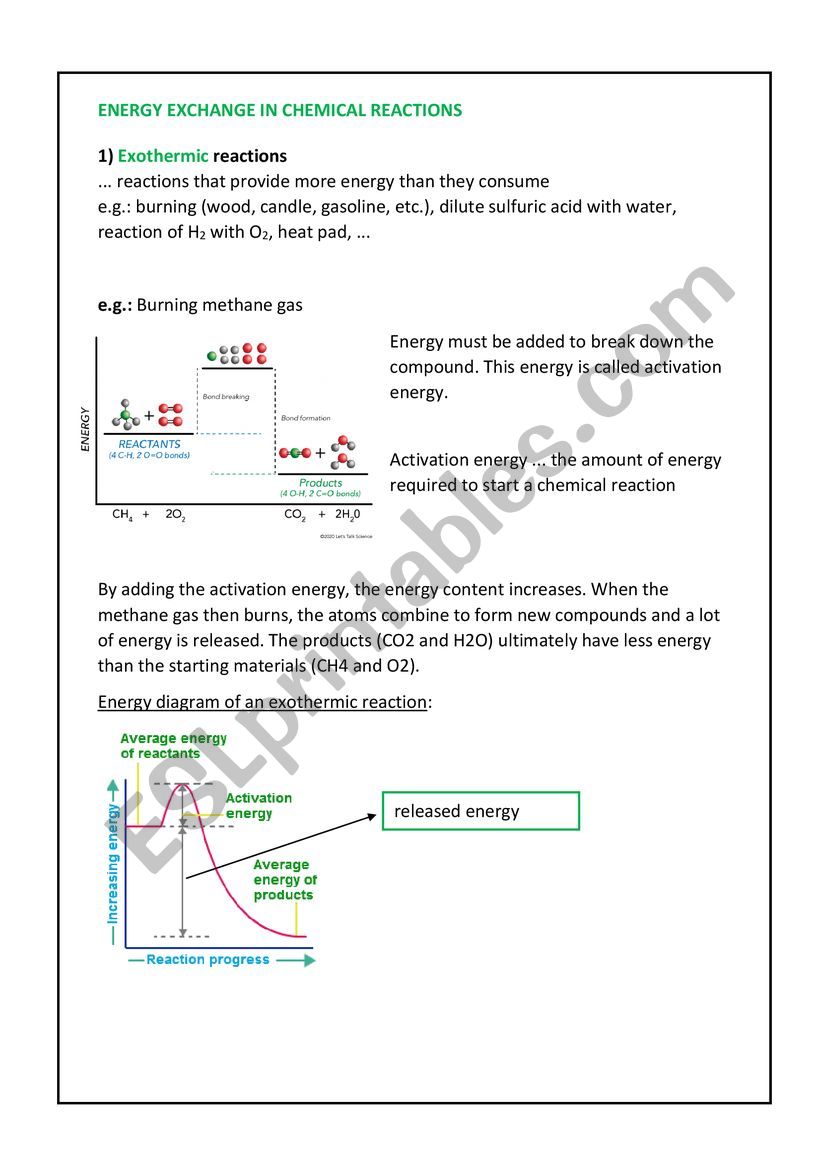
Image: www.eslprintables.com
This article delves into the captivating realm of endothermic and exothermic reactions, providing a clear understanding of their differences and similarities. We’ll also explore the accompanying worksheet answer key, designed to solidify your understanding of these fundamental concepts. Together, we’ll embark on a journey to unlock the secrets behind these powerful reactions and empower you with a deeper appreciation of the dynamic world of chemistry.
Delving into the Depths: Endothermic vs. Exothermic Reactions
Imagine a campfire, crackling with heat and light. This warmth is a classic example of an exothermic reaction, where energy is released into the surroundings. In contrast, an endothermic reaction absorbs energy from its surroundings, like the cool sensation of an ice pack.
At the heart of these reactions lies the concept of enthalpy, which represents the total energy contained within a system. In exothermic reactions, the products have lower enthalpy than the reactants, resulting in the release of energy. The energy released could be in the form of heat, light, or sound, as observed in the burning of wood in a campfire. Conversely, in endothermic reactions, the products have higher enthalpy than the reactants, requiring energy input from the surroundings to occur.
Beyond Definitions: Real-World Applications
These reactions aren’t confined to textbooks; they influence our daily lives in numerous ways. Let’s explore some real-world applications:
-
Cooking: Baking bread is an exothermic process. The heat released from the reaction of ingredients creates a delicious, warm loaf of bread. Melting chocolate, on the other hand, is an endothermic process, as it requires heat energy to transform the solid chocolate into a melted state.
-
Weather: The evaporation of water is an endothermic process. It absorbs heat from its surroundings, causing a cooling effect. Conversely, condensation, the formation of water droplets, is an exothermic process, releasing heat into the atmosphere.
-
Chemical Industry: Many industrial processes rely heavily on endothermic and exothermic reactions. For example, the production of ammonia, a vital ingredient in fertilizers, is an exothermic process that releases a significant amount of heat.
Diving Deeper: The Enthalpy Change and Its Importance
The energy change associated with a reaction is represented by the enthalpy change, denoted as ΔH. For exothermic reactions, ΔH is negative, indicating that energy is released. Conversely, for endothermic reactions, ΔH is positive, indicating energy absorption.
The enthalpy change is a crucial indicator of a reaction’s favorability. Reactions with negative enthalpy changes (exothermic) are generally spontaneous and release energy, making them more likely to occur. In contrast, reactions with positive enthalpy changes (endothermic) require energy input to proceed and are less spontaneous.
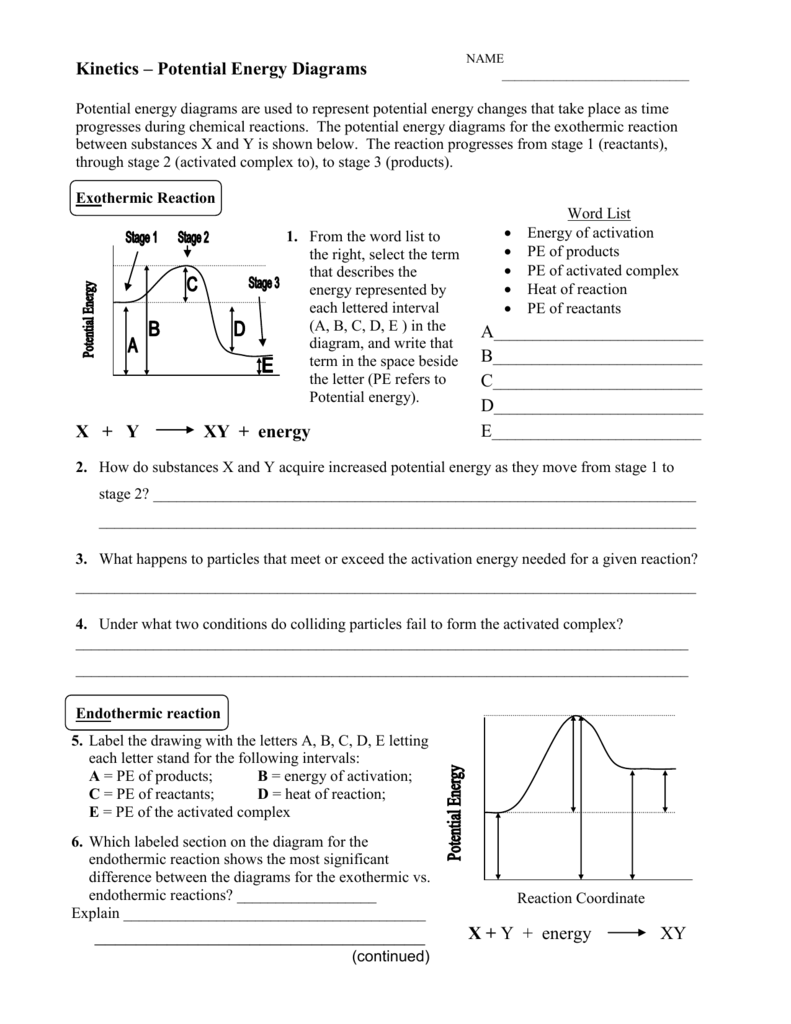
Image: studylib.net
A Guide to Understanding: The Worksheet Answer Key Explained
Now, let’s delve into the accompanying worksheet answer key to further solidify your understanding. Each question in the worksheet is designed to test your knowledge of the key concepts we’ve discussed—from identifying types of reactions to applying them to real-world scenarios.
The worksheet answers provide comprehensive explanations and detailed solutions to each question, allowing you to track your progress and identify any areas requiring further review.
-
Question 1: Classifying Reactions: This question challenges you to classify different reactions as endothermic or exothermic based on their descriptions. The answer key will clearly indicate the correct classification and provide reasoning based on the energy changes involved.
-
Question 2: Calculating Enthalpy Changes: This section asks you to calculate the enthalpy change of a reaction using given data. The answer key will walk you through the step-by-step calculations, highlighting the key formula and ensuring your grasp of the concept.
-
Question 3: Real-world Applications: This question delves into practical applications of endothermic and exothermic reactions. The answer key will provide detailed explanations of the processes involved, linking theoretical knowledge to real-life scenarios.
-
Question 4: Identifying Reactions from Real-life Observations: This section tests your understanding by asking you to identify the type of reactions based on real-life observations. The answer key will guide you through the process, highlighting the key indicators that distinguish endothermic and exothermic reactions.
Mastering the Concepts: Transforming Knowledge into Action
Understanding and applying the concepts of endothermic and exothermic reactions empowers you with a deeper understanding of the chemical processes that shape the world around us. By working through the worksheet and immersing yourself in the accompanying answer key, you’ll not only strengthen your knowledge but also develop critical thinking skills that are invaluable in scientific exploration.
This journey into the world of chemical reactions is not just about memorizing terms; it’s about developing a deeper appreciation for the fundamental principles that govern our world. So, embrace your curiosity, dive into the fascinating realm of endothermic and exothermic reactions, and unlock the secrets hidden within those seemingly simple chemical transformations.
Endothermic Reactions Vs Exothermic Reactions Worksheet Answer Key
Explore Further: Where to Find More Resources
Your journey of learning doesn’t end here! There’s a vast world of knowledge waiting to be explored. For further exploration, consider these resources:
-
Online Courses and Tutorials: Reputable websites like Khan Academy and Coursera offer engaging courses and tutorials on chemistry fundamentals, including endothermic and exothermic reactions.
-
Chemistry Textbooks: Explore introductory chemistry texts for detailed explanations and diverse examples related to these concepts.
-
Scientific Articles: Journals like the Journal of Chemical Education provide in-depth research articles on various chemical reactions, including endothermic and exothermic processes.
Share Your Thoughts: We’d love to hear your experiences with this topic! Share your insights, questions, or any discoveries you’ve made in the comments below. Let’s continue this exciting journey of exploration together!






