Have you ever wondered why some drinks fizz when you add a lemon wedge, or why your stomach churns after eating too much spicy food? The answers lie in the fascinating world of acids and bases, two fundamental chemical concepts that play a crucial role in our daily lives. This article will guide you through the intricacies of pH, a measure of acidity and basicity, and provide a comprehensive answer key for common acids/bases & pH worksheets.

Image: 2012books.lardbucket.org
Understanding the concepts of acids and bases is essential for comprehending various chemical reactions, biological processes, and even everyday activities like baking or gardening. This exploration will equip you with the knowledge to navigate the world of pH with confidence, empowering you to solve problems, conduct experiments, and make informed decisions in various scientific and practical contexts.
Delving into the Acidic and Basic World: A Primer on pH
What are Acids and Bases?
Imagine a chemical substance with a distinct sour taste that releases hydrogen ions (H+) in water. That’s an **acid**. The more hydrogen ions it releases, the stronger the acid. On the other hand, a chemical substance with a bitter taste that releases hydroxide ions (OH-) in water is known as a **base**.
The pH Scale: A Quantified Measure of Acidity & Basicity
The pH scale provides a numerical framework for measuring the acidity or basicity of a solution. It ranges from 0 to 14, with 7 being neutral. Solutions with a pH less than 7 are acidic, while those with a pH greater than 7 are basic or alkaline.
The pH scale is logarithmic, meaning that each whole number change represents a tenfold change in acidity or basicity. So, a solution with a pH of 3 is ten times more acidic than a solution with a pH of 4. This logarithmic relationship further underscores the importance of pH in understanding chemical reactions.
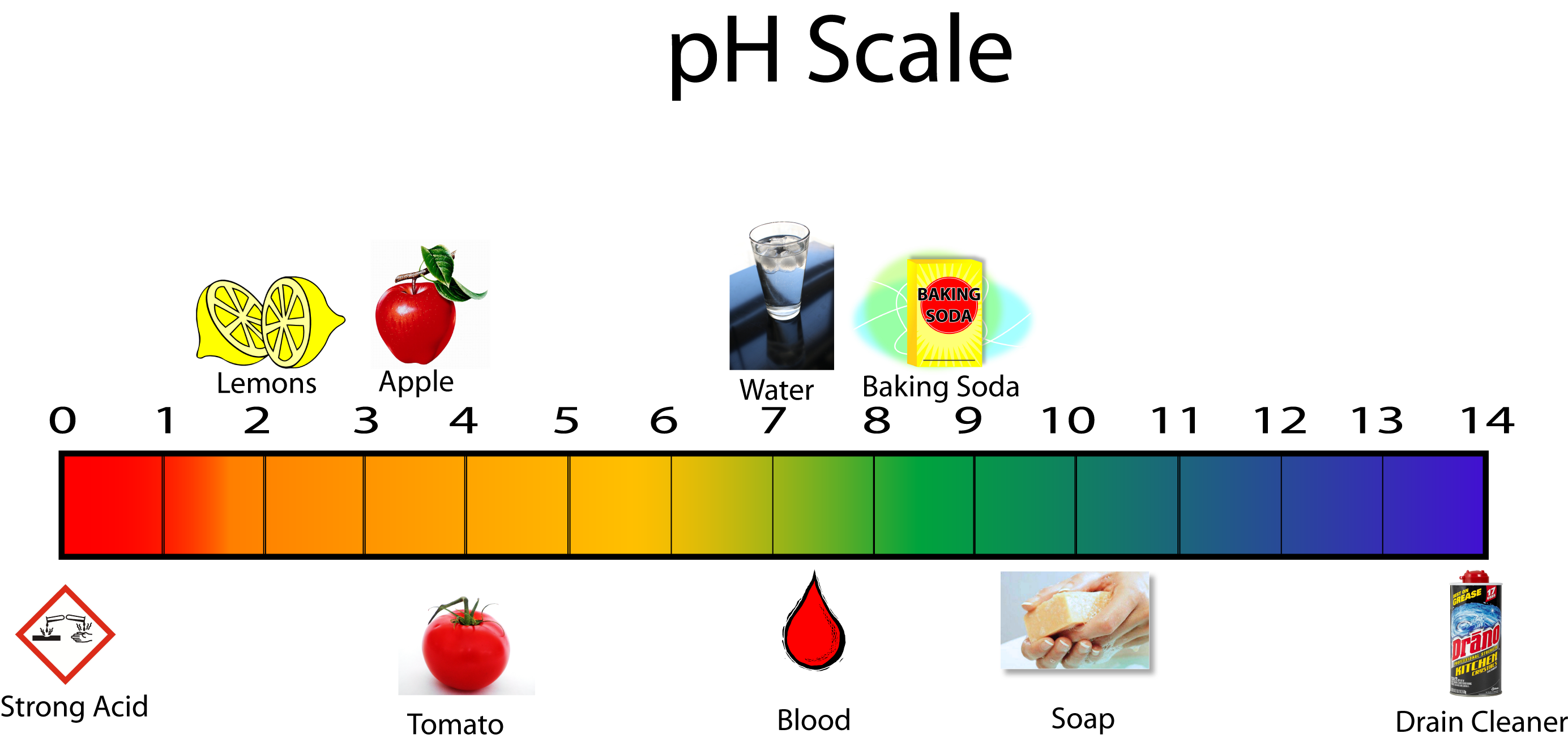
Image: mchsscience.weebly.com
Common Examples of Acids and Bases: Encounters in Everyday Life
Acids and bases are ubiquitous in our daily lives, often influencing the taste, texture, and functionality of substances we encounter. Let’s explore some common examples:
- Acids: Lemon juice (citric acid), vinegar (acetic acid), stomach acid (hydrochloric acid), battery acid (sulfuric acid)
- Bases: Baking soda (sodium bicarbonate), soap (sodium hydroxide), ammonia (ammonium hydroxide), antacids (calcium carbonate)
Real-World Applications: pH is Everywhere
The concept of pH extends beyond the confines of the chemistry lab, influencing a wide array of real-world applications:
- Agriculture: pH levels in soil affect the availability of nutrients for plants. Farmers carefully monitor and adjust soil pH to ensure optimum crop growth.
- Medicine: Maintaining the pH balance of blood and other bodily fluids is crucial for vital functions. Medications and medical treatments often involve pH considerations.
- Food and Beverage: pH plays a vital role in food preservation, taste, and texture. For instance, the pH of pickles and jams helps prevent spoilage.
- Environmental Protection: pH levels in water bodies can impact aquatic life and ecosystems. Maintaining appropriate pH levels is essential for environmental protection.
Navigating the Waters of pH: Key Concepts and Techniques
The pH Formula: Calculating the Acidity or Basicity
The pH formula is a powerful tool for quantifying acidity and basicity. It is derived from the concentration of hydrogen ions (H+) in a solution. The formula is expressed as follows:
pH = -log[H+]
Where [H+] represents the hydrogen ion concentration in moles per liter (mol/L). This formula is used to calculate the pH of a solution, given the hydrogen ion concentration. Conversely, you can determine the hydrogen ion concentration if the pH is known.
Indicators: Colorful Clues to pH Levels
pH indicators are substances that change color depending on the pH of a solution. These indicators help us visually determine the acidity or basicity of solutions without the need for complicated calculations. Examples of common indicators include:
- Litmus paper: Turns red in acidic solutions and blue in basic solutions.
- Phenolphthalein: Colorless in acidic solutions and pink in basic solutions.
- Methyl orange: Red in acidic solutions, orange in neutral solutions, and yellow in basic solutions.
Titration: Precisely Measuring pH Changes
Titration is a chemical technique used to determine the concentration of an unknown solution by reacting it with a solution of known concentration. In acid-base titration, a known volume of an acid or base of known concentration is added to a solution of unknown concentration, until a specific pH is reached. This process allows for the precise determination of the unknown solution’s concentration. This technique is widely used in chemistry, biology, and other fields to analyze and quantify pH changes in various processes.
Answering the Call: A Comprehensive Acids/Bases & pH Worksheet Answer Key
Now, let’s move to the practical application of these concepts. Here is a comprehensive answer key for the most common acids/bases and pH worksheet questions:
**1. What are the properties of acids? **
Answer: Acids have a sour taste, release hydrogen ions (H+) in water, react with bases to form salt and water, and turn blue litmus paper red.
**2. What are the properties of bases?**
Answer: Bases have a bitter taste, release hydroxide ions (OH-) in water, react with acids to form salt and water, and turn red litmus paper blue.
**3. What is the pH scale?**
Answer: The pH scale measures the acidity or basicity of a solution. It ranges from 0 to 14, with 7 being neutral. A pH less than 7 indicates an acid, while a pH greater than 7 indicates a base.
**4. What is the relationship between hydrogen ion concentration and pH?**
Answer: The pH of a solution is inversely proportional to the hydrogen ion concentration. Higher hydrogen ion concentrations correspond to lower pH values (more acidic), and lower hydrogen ion concentrations correspond to higher pH values (more basic).
**5. What is an indicator?**
Answer: An indicator is a substance that changes color depending on the pH of a solution. They help visually determine the acidity or basicity of a solution.
**6. What is titration?**
Answer: Titration is a technique used to determine the concentration of an unknown solution by reacting it with a solution of known concentration. It is commonly used in chemistry to analyze and quantify pH changes in various processes.
**7. What is the pH of a neutral solution? **
Answer: The pH of a neutral solution is 7. This indicates equal amounts of hydrogen and hydroxide ions.
**8. Identify the following solutions as acidic, basic, or neutral: Lemon juice, vinegar, soap, baking soda, coffee, water, stomach acid, antacids. **
Answer:
Acidic: Lemon juice, vinegar, coffee, stomach acid
Basic: Soap, baking soda, antacids.
Neutral: Water
**9. What is the pH of a solution with a hydrogen ion concentration of 1 x 10^-4 mol/L?**
Answer: pH = -log[H+] = -log(1 x 10^-4) = 4. The solution is acidic.
**10. What is the hydrogen ion concentration of a solution with a pH of 9?**
Answer: [H+] = 10^-pH = 1 x 10^-9 mol/L. The solution is basic.
Acids/Bases & Ph Worksheet Answer Key
Embracing the Power of pH: A Call to Action
The journey into the realm of acids, bases, and pH has revealed the intricate workings of chemical reactions and their influence on the world around us. Armed with this knowledge, you can now tackle pH-related worksheets with confidence, apply these concepts to real-world problems, and delve deeper into the fascinating world of chemistry. Remember, learning is a continuous process, so don’t hesitate to explore further resources, experiment with different indicators, and let your curiosity guide you. The possibilities for understanding and utilizing the power of pH are endless!






