Have you ever pondered the seemingly magical connection between the weight of a substance and the space it occupies? It’s a question that has fascinated scientists for centuries, leading to the development of groundbreaking concepts like the mole, a fundamental unit in chemistry that quantifies the amount of matter. This article delves into the fascinating world of 10.2 mole mass and mole volume relationships, unraveling the intricate dance between these fundamental concepts.
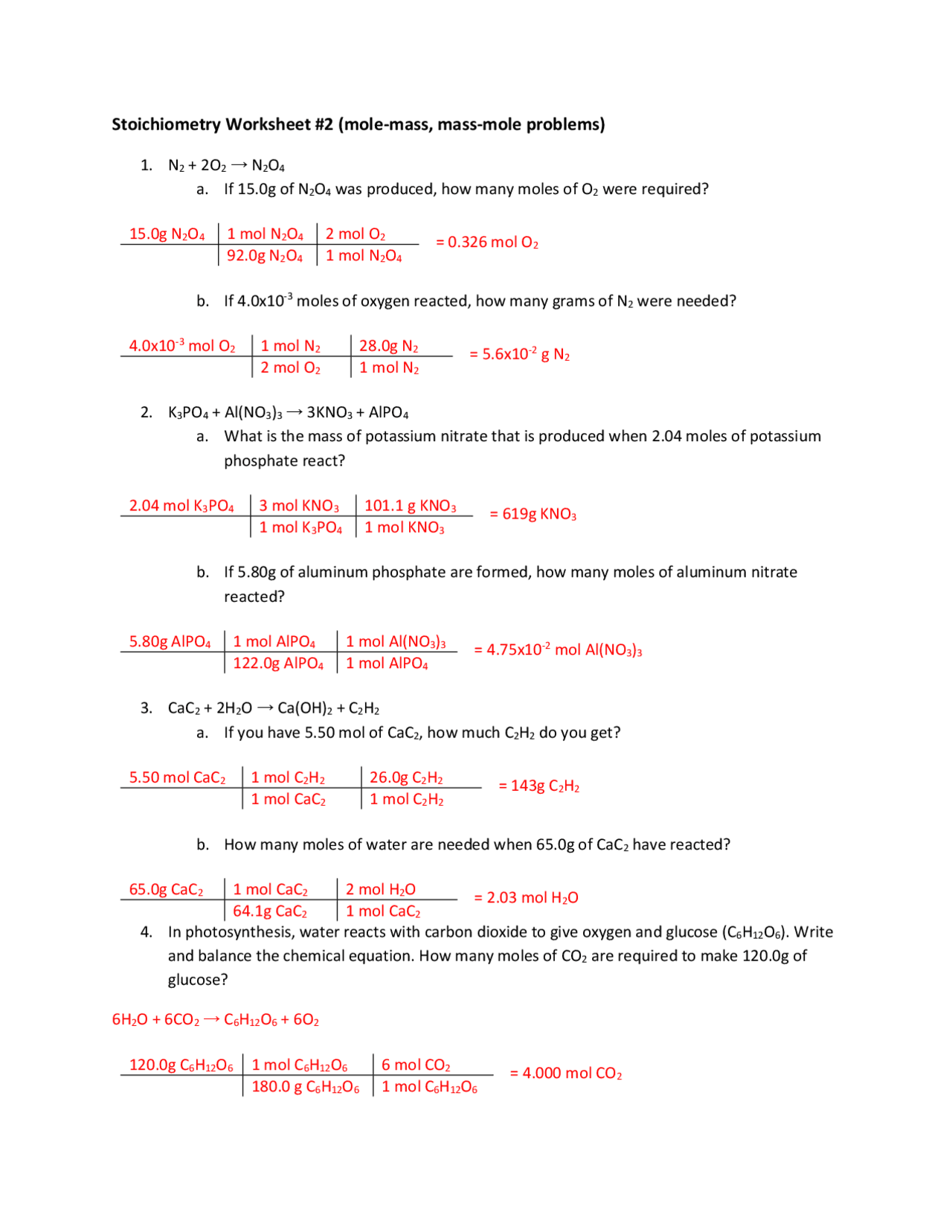
Image: learningcampusabend.z4.web.core.windows.net
Understanding the relationship between moles, mass, and volume is crucial for anyone seeking to master chemistry. Whether you’re a student navigating your first chemistry course or a seasoned scientist conducting complex experiments, these concepts serve as the foundation for quantitative chemistry. Armed with this knowledge, you can unlock the secrets of how much substance is in a given volume and vice versa, paving the way for precise calculations and reliable experimental outcomes.
The Mole: A Key to Understanding Matter
Defining the Mole: A Chemist’s Dozen
The mole, a unit often compared to a chemist’s dozen, denotes a specific quantity of matter. Specifically, one mole is equal to 6.022 × 1023 particles, a number known as Avogadro’s number. Imagine a mole as a convenient package containing an enormous number of atoms or molecules, allowing us to work with these tiny entities on a much larger scale.
Connecting the Mole to Mass
The beauty of the mole lies in its ability to connect the microscopic world of atoms and molecules to the macroscopic world we experience. The molar mass, a fundamental property of a substance, represents the mass of one mole of that substance. This relationship is expressed by the simple equation: mass (g) = moles (mol) × molar mass (g/mol). This equation allows us to seamlessly convert between the mass of a substance and the number of moles present.

Image: www.slideshare.net
Volume: The Space Occupied by Matter
Introducing Volume: A Matter of Space
Volume, the amount of three-dimensional space occupied by a substance, plays a critical role in understanding the physical properties of matter. For gases, volume is directly related to the number of moles present. This relationship is formalized by the Ideal Gas Law, which states that the volume of an ideal gas is directly proportional to the number of moles, temperature, and inversely proportional to pressure.
Utilizing Volume to Determine Moles
The relationship between volume and moles is particularly important in gas chemistry. By knowing the volume of a gas, its temperature, and pressure, we can determine the number of moles present using the Ideal Gas Law: PV = nRT, where P represents pressure, V is volume, n is the number of moles, R is the ideal gas constant, and T is temperature.
The 10.2 Connection: Unveiling the Link
The 10.2 relationship refers to the specific interplay between moles, mass, and volume in certain chemical contexts. This relationship is particularly relevant in situations involving gases, where volume plays a crucial role. For example, in stoichiometry calculations involving gas reactions, the 10.2 relationship provides a valuable tool for converting between mass, volume, and number of moles.
Example: Applying the 10.2 Relationship
Let’s say we have a reaction where one mole of a gas produces two moles of another gas at a specific temperature and pressure. Using the Ideal Gas Law, we can determine the volume each gas occupies. We can then use the 10.2 relationship to calculate the mass of each reactant and product based on the known volume and molar mass. This exemplifies how the relationship simplifies calculations and provides a deeper understanding of chemical processes.
Expanding the Horizon: Real-World Applications
The 10.2 mole mass and volume relationships are not simply theoretical concepts; they find practical applications in numerous fields. Industries such as pharmaceuticals, chemical engineering, and environmental science rely on these principles for efficient production, precise analysis, and informed decision-making.
For example, pharmaceutical companies use these relationships to calculate the exact amount of reactants needed to synthesize specific medications, ensuring the production of safe and effective drugs. Chemical engineers utilize these concepts to optimize industrial processes, maximizing efficiency and minimizing waste. In environmental science, these relationships are critical for evaluating air and water quality, ensuring compliance with environmental regulations.
Beyond the Laboratory: Everyday Applications
The principles of mole mass and volume relationships extend beyond the confines of a laboratory, impacting our everyday lives. Baking requires understanding the relationship between the amount of ingredients, their volume, and their impact on the final product. Even driving a car utilizes these concepts, as the amount of fuel combusted directly relates to the volume of exhaust gases produced.
10.2 Mole Mass And Mole Volume Relationships
Conclusion: Embracing the Power of Chemistry
The seemingly simple connection between moles, mass, and volume unlocks a vast universe of chemical understanding. By comprehending these relationships, we can delve into the heart of chemical reactions, predict outcomes, and design new materials with specific properties. This exploration has unveiled the fascinating dance between these fundamental concepts, demonstrating their vital role in both scientific research and everyday life. You are now equipped to embark on your journey of discovery, applying this knowledge to further your understanding of the world around you. From scientific experimentation to everyday observations, the 10.2 mole mass and volume relationships offer a powerful lens through which to view the wonders of chemistry.






