Have you ever wondered why water molecules are bent, while methane molecules are perfectly tetrahedral? The answer lies in a fundamental concept in chemistry known as VSEPR, short for Valence Shell Electron Pair Repulsion Theory. This theory, which forms the cornerstone of predicting molecular shapes, states that electron pairs around a central atom arrange themselves to minimize repulsions, leading to specific geometric shapes. The Phet VSEPR activity, a valuable online tool, provides an interactive and engaging way to explore these molecular geometries and build a deeper understanding of chemical bonding.

Image: www.coursehero.com
In this comprehensive guide, we will delve into the world of molecular shapes, exploring the VSEPR theory, its applications, and the role of the Phet VSEPR activity in visualising and understanding these concepts. We will break down the theory, analyze different molecular shapes, and provide insights into how the Phet activity can enhance your understanding of this crucial aspect of chemistry.
Understanding VSEPR Theory
The Foundation of Molecular Shapes
VSEPR theory is a fundamental principle in chemistry that governs the three-dimensional arrangement of atoms in molecules. It’s built on the idea that electron pairs, both bonding and non-bonding, surrounding a central atom repel each other and strive to maximize their distance. This repulsion, a result of electrostatic forces, leads to specific geometric configurations that define the molecule’s shape.
The Role of Lone Pairs
VSEPR theory emphasizes the significance of lone pairs, which are non-bonding electron pairs located on the central atom. These lone pairs exert greater repulsive forces than bonding pairs due to their greater electron density. As a result, lone pairs “push” bonding pairs further apart, influencing the overall molecular geometry. For instance, the presence of lone pairs in water molecules leads to a bent shape, as they repel the bonding pairs, causing a deviation from a linear arrangement.
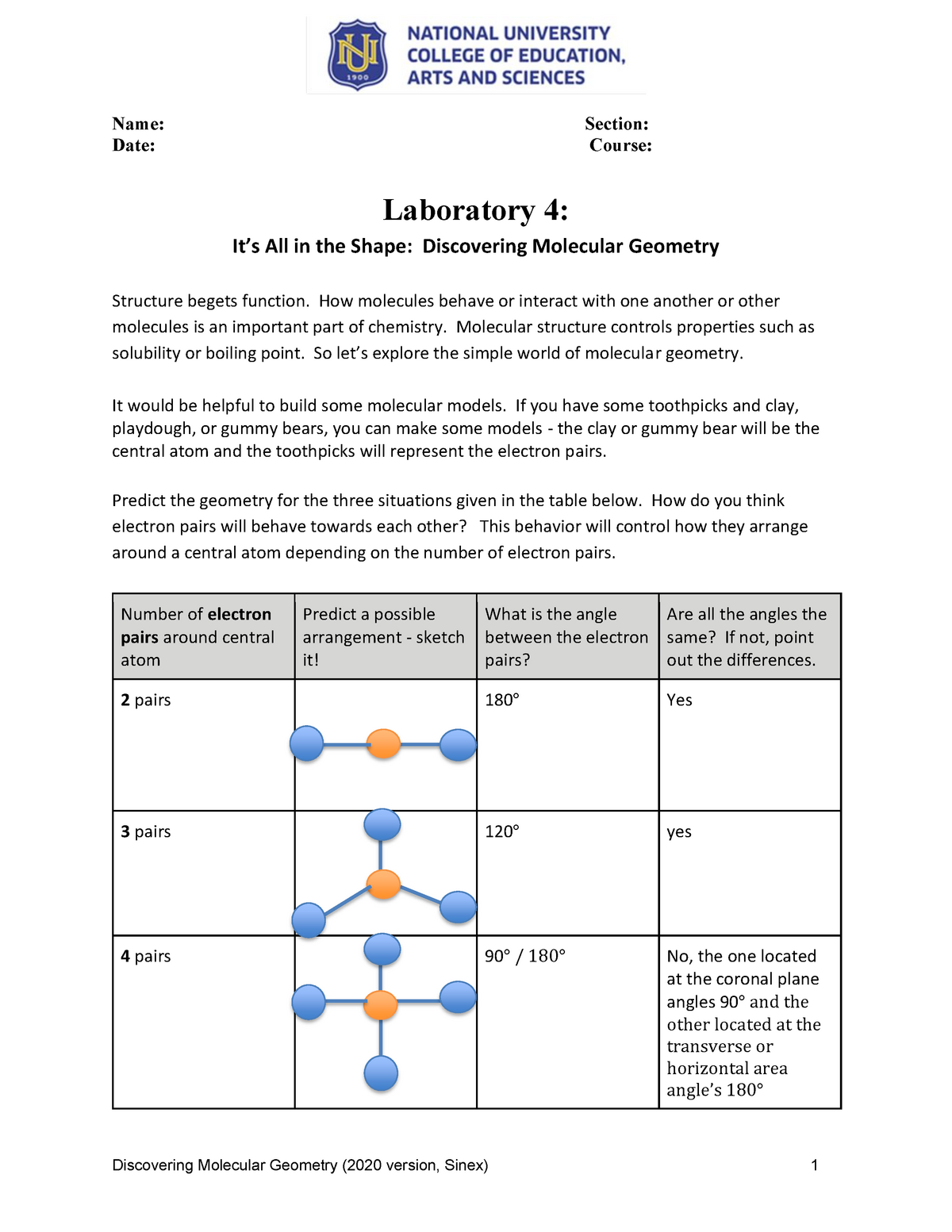
Image: www.studocu.com
Predicting Molecular Shapes
The VSEPR theory provides a systematic framework for predicting molecular shapes based on the number of electron pairs around the central atom. The key to this prediction lies in the concept of “electron domains.” An electron domain encompasses any region around the central atom where electrons are localized, including bonding pairs and lone pairs. By considering the arrangement of these domains, we can determine the molecular shape.
Exploring Molecular Shapes with Phet
The Phet VSEPR Activity: A Hands-On Approach
The Phet VSEPR activity, developed by the PhET Interactive Simulations project, provides a highly interactive and engaging way to explore molecular shapes. This online tool allows you to build molecules by adding atoms and bonds, visualize the electron arrangement around the central atom, and predict the resulting molecular geometry. The activity is designed to be user-friendly, making learning about VSEPR both enjoyable and effective.
Key Features of the Phet VSEPR Activity
- Interactive Molecule Building: Add atoms, create bonds, and modify the molecule’s structure to investigate the impact of different arrangements on molecular geometry.
- Electron Domain Visualization: The activity vividly displays the electron domains surrounding the central atom, highlighting the repulsive forces that govern molecular shape.
- Shape Prediction: Based on the number of electron domains, the activity predicts the molecular shape and provides a clear representation of the geometric configuration.
- User-Friendly Interface: The activity is designed for intuitive exploration, enabling learners of all backgrounds to engage with VSEPR concepts easily.
Key Molecular Shapes and their VSEPR Predictions
Linear Molecules (AX2)
This shape is observed when the central atom (A) is surrounded by two bonding pairs (X). The electron domains are arranged in a linear fashion, resulting in a bond angle of 180°. Examples include carbon dioxide (CO2) and beryllium chloride (BeCl2).
Trigonal Planar Molecules (AX3)
These molecules feature a central atom with three bonding pairs arranged in a triangular plane. The bond angles are 120°, and the molecule is flat. Boron trifluoride (BF3) and formaldehyde (H2CO) are typical examples.
Tetrahedral Molecules (AX4)
This geometry is characterized by a central atom with four bonding pairs arranged in a tetrahedral shape. The bond angles are approximately 109.5°, creating a three-dimensional structure. Examples include methane (CH4) and carbon tetrachloride (CCl4).
Trigonal Pyramidal Molecules (AX3E)
In this case, the central atom has three bonding pairs and one lone pair, leading to a pyramidal shape with a triangular base. The bond angles are slightly less than 109.5° due to the repulsion from the lone pair. Ammonia (NH3) is a classic example.
Bent Molecules (AX2E2)
When two lone pairs are present around the central atom with two bonding pairs, the molecular geometry is bent. The bond angle is smaller than in a tetrahedral structure, as the lone pairs repel the bonding pairs more strongly. Water (H2O) is the prime example of this shape.
The Importance of Molecular Shapes
Understanding molecular shapes is not just a theoretical exercise; it carries immense practical importance in various fields:
Chemistry and Reactivity
Molecular shapes dictate a molecule’s reactivity. The spatial arrangement of atoms influences how molecules interact with each other, impacting their ability to form bonds, undergo reactions, and participate in complex biochemical processes.
Biology and Medicine
Molecular shapes are crucial in biological systems. The specific shape of enzymes, proteins, and nucleic acids determines their functions, enabling them to catalyze reactions, transport molecules, and govern genetic information. Understanding molecular shapes is essential in drug design, as it allows scientists to develop drugs that fit specific targets within the body.
Materials Science
The properties of materials are heavily dependent on the shape of their constituent molecules. By controlling molecular shapes, scientists can tailor materials for specific applications in areas like polymers, catalysts, and semiconductors.
Beyond the Basics: Advanced VSEPR Concepts
While the core concepts of VSEPR theory establish a foundation for understanding molecular shapes, advanced VSEPR concepts allow for deeper insights into more complex structures. These include:
Expanded Octet
In some molecules, the central atom can accommodate more than eight electrons around it. This expansion of the octet is possible for elements in periods three and beyond, due to the availability of vacant d orbitals. This expansion can lead to different molecular geometries, such as trigonal bipyramidal or octahedral shapes.
Hybridization
Hybridization refers to the mixing of atomic orbitals to form new hybrid orbitals. The hybridization of orbitals can significantly influence molecular shapes, particularly in cases involving double or triple bonds, leading to deviations from the expected VSEPR geometry.
Phet Molecular Shapes Vsepr Activity Answer Key
https://youtube.com/watch?v=r5CeFYcIM3g
Conclusion
Understanding molecular shapes is essential for comprehending the behavior of molecules in various disciplines. VSEPR theory provides a powerful framework for predicting geometric arrangements, while the Phet VSEPR activity offers an interactive and engaging way to learn and visualize these concepts. By mastering these principles, you can gain a deeper appreciation for the intricate world of chemistry and its impact on our world. Continue exploring beyond this guide to delve deeper into the fascinating realm of molecular shapes, their significance, and their applications in different fields.






