Have you ever stared at a gas laws color by number worksheet, feeling overwhelmed by the intricate formulas and puzzling numbers? Fear not, intrepid explorer of the gaseous realm! This journey will equip you with the knowledge to conquer those color-coded challenges and unlock the mysteries hidden within each equation. We will journey through the fascinating world of gas laws, demystifying the principles that govern the behavior of these elusive entities. By the end of this exploration, you’ll be able to not only solve your gas laws color by number worksheets but also understand the profound impact these laws have on our everyday lives.

Image: shirathemaiden.blogspot.com
The study of gas laws is not confined to the realm of textbooks and worksheets. Imagine a hot air balloon gracefully soaring through the sky, or a scuba diver exploring the underwater world. These thrilling adventures are made possible by the meticulous application of these fundamental laws. Whether you’re a budding scientist, a curious student, or simply someone seeking to understand the invisible forces that shape our world, join us as we embark on this enlightening expedition into the fascinating world of gas laws.
The Fundamental Principles: Setting the Stage for Understanding
Before we dive into the colorful complexities of your gas laws color by number worksheet, let’s lay down the foundations of our knowledge. You see, gases are not just wispy, intangible substances but rather collections of tiny particles called molecules. These molecules are constantly in motion, colliding with each other and the walls of their container. This dynamic dance of molecules leads to the measurable properties of gases, such as pressure, volume, and temperature—the very elements that will unlock the secrets of your color-by-number puzzle.
The Pressure Puzzle: A Tale of Constant Motion
Imagine a swarm of bees buzzing around a honeycomb. Each bee, representing a gas molecule, constantly bumps into the walls of their hive, representing the container holding the gas. The force of these collisions creates pressure, much like the bees’ jostling creates a buzz. The more bees, the higher the pressure; similarly, the more gas molecules, the greater the pressure inside the container.
In the world of gas laws, pressure is often represented by the symbol ‘P’ and measured in units like atmospheres (atm) or millimeters of mercury (mmHg). The higher the pressure, the more tightly packed the gas molecules become, and the more force they exert on the container walls.
Volume’s Expansion: A World of Unlimited Possibilities
Now imagine our beehive expands, providing more space for the bees to fly around. This increased space translates to a larger volume, giving the bees more freedom of movement. Similarly, the volume of a gas is determined by the space it occupies. It is often symbolically represented by ‘V’ and measured in units like liters (L) or milliliters (mL).
The greater the volume of a gas, the more space its molecules have to move around, resulting in fewer collisions with the container walls and, consequently, lower pressure. This inverse relationship between volume and pressure forms the foundation of one of the most fundamental gas laws—Boyle’s Law.
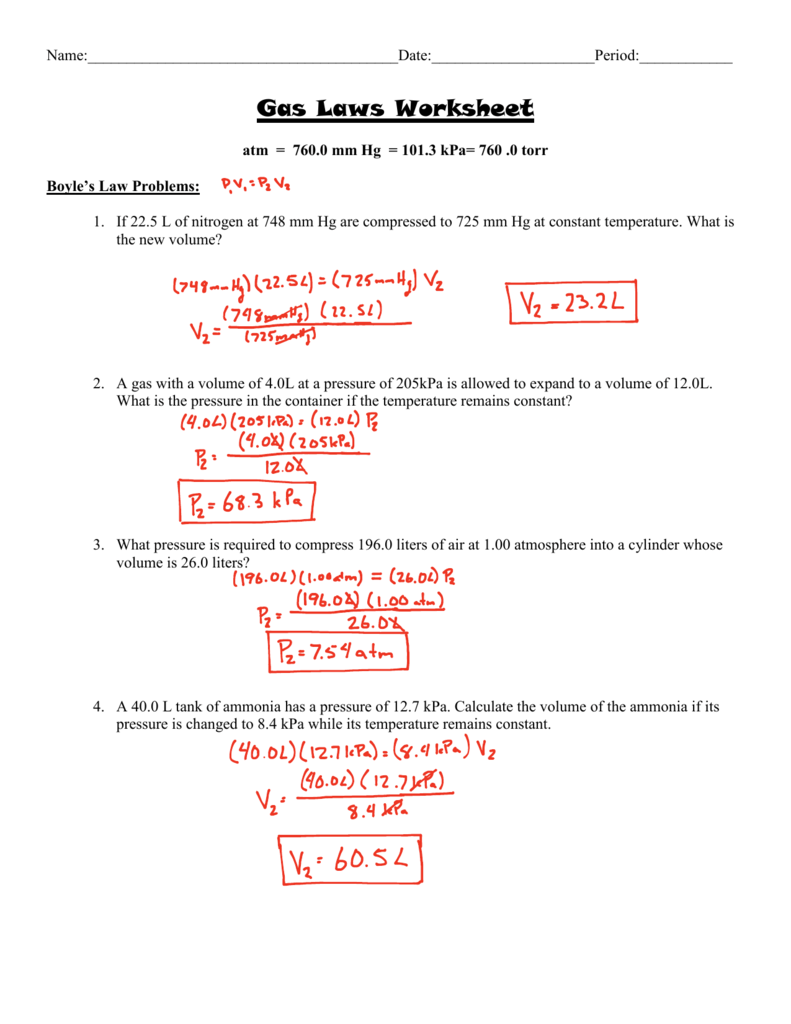
Image: studylib.net
The Heat of the Matter: Unlocking the Power of Temperature
Imagine you heat up the beehive. The bees become more active, buzzing around with greater energy and speed. This increased energy translates to a higher temperature, just as heating the gas container will increase the kinetic energy of its molecules. Temperature, represented by ‘T’ and measured in units like Celsius (°C) or Kelvin (K), plays a crucial role in governing the behavior of gases.
Higher temperatures mean faster-moving molecules, which in turn lead to more frequent and forceful collisions with the container walls, resulting in increased pressure. This direct relationship between temperature and pressure is a key aspect of Gay-Lussac’s Law.
The Colorful World of Gas Laws: Solving Your Color by Number Worksheet
With a grasp of the fundamental principles of pressure, volume, and temperature, we are ready to tackle the colorful complexities of your gas laws color by number worksheet. Each color on your worksheet represents a specific gas law, and the corresponding numbers represent the values for the variables involved. By understanding the relationship between pressure, volume, and temperature, you can solve for the missing value and reveal the hidden colors.
Boyle’s Law: A Tale of Inverse Proportions
Boyle’s Law governs the relationship between pressure and volume at a constant temperature. It tells us that as pressure increases, volume decreases, and vice versa. This inverse relationship is represented by the equation:
P₁V₁ = P₂V₂
This equation states that the product of the initial pressure (P₁) and initial volume (V₁) is equal to the product of the final pressure (P₂) and final volume (V₂), under conditions of constant temperature. On your color by number worksheet, you might find a set of numbers representing pressure and volume changes within a specific scenario. Using Boyle’s Law, you can calculate the missing value and unveil the corresponding color.
Charles’s Law: Expanding Horizons with Temperature
Charles’s Law focuses on the relationship between volume and temperature at constant pressure. It states that as temperature increases, volume also increases, forming a direct relationship. The equation representing Charles’s Law is:
V₁/T₁ = V₂/T₂
This equation indicates that the ratio of initial volume (V₁) to initial temperature (T₁) is equal to the ratio of final volume (V₂) to final temperature (T₂), provided pressure remains constant. On your color-by-number worksheet, you’ll find scenarios where temperature and volume change while pressure remains constant. By applying Charles’s Law, you can calculate the missing value and reveal another color on your masterpiece.
Gay-Lussac’s Law: The Heat of the Pressure
Gay-Lussac’s Law delves into the relationship between pressure and temperature at constant volume. It states that as temperature increases, pressure also increases proportionally. The equation for Gay-Lussac’s Law is:
P₁/T₁ = P₂/T₂
This equation highlights that the ratio of initial pressure (P₁) to initial temperature (T₁) remains equal to the ratio of final pressure (P₂) to final temperature (T₂), with volume staying constant. Your color-by-number worksheet might present scenarios with varying pressure and temperature while volume remains unchanged. By applying Gay-Lussac’s Law, you can solve for the missing component and reveal the concealed color.
The Combined Gas Law: Merging Forces for Comprehensive Understanding
Combining these three laws creates the comprehensive Combined Gas Law, which encapsulates the relationship between pressure, volume, and temperature for a fixed amount of gas. It is represented by the equation:
(P₁V₁)/T₁ = (P₂V₂)/T₂
This equation demonstrates that the product of initial pressure (P₁) and initial volume (V₁) divided by initial temperature (T₁) remains equal to the product of final pressure (P₂) and final volume (V₂) divided by final temperature (T₂). For complex scenarios on your color-by-number worksheet, where all three variables—pressure, volume, and temperature—change, the Combined Gas Law provides the necessary framework to calculate the missing value and reveal the corresponding color.
The Ideal Gas Law: Reaching for Perfection
The Ideal Gas Law takes our understanding of gas behavior to a new level by introducing the concept of moles, a unit that represents a fixed amount of substance. It provides a comprehensive equation that relates pressure, volume, temperature, and moles of a gas:
PV = nRT
This equation, where P represents pressure, V stands for volume, n signifies the number of moles, R is the ideal gas constant, and T is temperature, encapsulates the behavior of an ideal gas—a theoretical gas that perfectly adheres to these relationships. While real gases do not perfectly fit the Ideal Gas Law, it serves as a powerful model for predicting and understanding the behavior of gases under various conditions.
Beyond the Worksheet: The Real-World Impact of Gas Laws
The gas laws are not just abstract scientific principles confined to textbooks and worksheets. They form the foundation for understanding and controlling various phenomena, influencing our daily lives in countless ways.
Weather Forecasting: Predicting the Winds of Change
Weather forecasting, an integral part of our daily life, relies heavily on the principles of gas laws. Temperature gradients, pressure differences, and wind patterns are all governed by these laws, allowing meteorologists to predict storms, track hurricanes, and understand the complex dynamics of Earth’s atmosphere.
Deep-Sea Exploration: Diving into the Depths of Knowledge
Underwater exploration, whether for scientific research or recreational diving, necessitates understanding the behavior of gases at different depths. Pressure increases significantly as one descends into the ocean, a phenomenon governed by the Ideal Gas Law. Divers must carefully monitor their air supply and decompression procedures to ensure safe ascents, thanks to the knowledge gleaned from these fundamental laws.
Engineering Marvels: Building the Future
The principles of gas laws are crucial for engineering feats, shaping everything from the design of aircraft to the development of efficient combustion engines. Understanding the behavior of gases under pressure and temperature changes is paramount for designing safe and efficient systems, ensuring optimal performance and minimizing risks.
Medical Innovations: Breathing Life into Technology
Medical applications also benefit tremendously from our understanding of gas laws. Respiratory therapy devices, such as ventilators, rely on these laws to deliver precise oxygenation to patients. From treating lung conditions to administering anesthesia, gas laws play a critical role in advancing medical technology and improving patient care.
Space Exploration: Reaching for the Stars
Space exploration, a testament to human ingenuity, is deeply rooted in our understanding of gas laws. Rockets rely on the controlled combustion of fuel, governed by the principles of pressure and temperature. The vacuum of space presents unique challenges for spacecraft design, necessitating careful consideration of gas expansion and behavior in extreme conditions.
Gas Laws Color By Number Answers
A Journey of Discovery: Unleashing the Power of Knowledge
As you navigate your way through your gas laws color by number worksheet, remember that each color you unveil is not just a simple visual change but a testament to the power of knowledge. These seemingly simple equations hold the keys to understanding the complex and vital world of gases, impacting countless aspects of our lives.
So, explore the fascinating world of gas laws with curiosity and passion. Don’t just focus on solving your color by number puzzle but delve deeper, explore the real-world applications, and marvel at the scientific principles that shape our existence. Remember, learning is not just about memorizing equations but about unlocking a vast world of knowledge and understanding, empowering you to see the world in a whole new light.






