Imagine peering into the heart of an atom, a tiny universe teeming with unseen forces. For centuries, scientists have been unraveling the mysteries of this fundamental building block of matter. One breakthrough that dramatically shifted our understanding was the Bohr model of the hydrogen atom. This model, proposed by Niels Bohr in 1913, not only offered a visual representation of atomic structure but also provided a groundbreaking explanation for the spectral lines emitted by hydrogen. Today, we can explore this landmark scientific concept in an interactive and engaging way through digital simulations called Gizmos.

Image: cronodon.com
In this article, we’ll embark on a journey into the captivating realm of the Bohr model and hydrogen atom. We’ll delve into the historical context, explain the key principles, and demonstrate how Gizmos can bring this abstract concept to life. You’ll gain a clearer understanding of the model’s strengths and limitations, as well as its profound implications for our understanding of light, energy levels, and the very nature of matter itself.
A Journey Through the Atom: Understanding the Bohr Model
The Bohr model, a revolutionary leap in atomic theory, presented a novel way of visualizing the hydrogen atom. It depicted the atom as a tiny solar system with a positively charged nucleus at the center, much like the sun, and a single negatively charged electron orbiting the nucleus in a circular path. The key insight of the model lay in its explanation of the discrete energy levels that the electron could occupy. Instead of continuously emitting radiation as it circled the nucleus, as classical physics predicted, the Bohr model postulated that the electron could only exist in specific energy levels, also known as quantized energy states.
The model further explained that when an electron transitioned from a higher energy level to a lower one, it emitted a photon of light with a specific frequency corresponding to the energy difference between the two levels. Conversely, if a photon with the right amount of energy struck the hydrogen atom, it could excite the electron to a higher energy level. This quantum leap explained the distinct spectral lines observed in the light emitted by hydrogen gas, a phenomenon that had baffled scientists for decades.
Beyond the Basics: Exploring the Bohr Model with Gizmos
Gizmos, interactive simulations that bring learning to life, provide an immersive and engaging way to understand the Bohr model and its applications. These digital tools allow users to manipulate variables and observe the resulting changes, fostering a deeper understanding of scientific concepts.
For instance, one Gizmo might allow you to experiment with the energy levels within a hydrogen atom. You could visualize the different energy states, the transitions between them, and the resulting emitted photons. Another Gizmo could demonstrate the effects of applying a magnetic field to the atom, revealing its quantized angular momentum. Through these interactive experiences, you can explore the underlying principles of the Bohr model not just as abstract theories, but as tangible phenomena with real-world consequences.
A Glimpse into the Quantum World: The Bohr Model’s Legacy
While the Bohr model proved remarkably successful in explaining the spectral properties of hydrogen, it had its limitations. It failed to accurately predict the spectra of atoms with multiple electrons and couldn’t account for the fine structure of spectral lines observed in experiments. However, its impact on our understanding of the atom was profound. It laid the groundwork for quantum mechanics, a more sophisticated theory that provided a more accurate description of atomic structure and behavior.
The Bohr model also helped to solidify the concept of quantization, the idea that energy, momentum, and other physical quantities exist in discrete packets rather than continuous values. This principle has become fundamental to modern physics, with applications in fields ranging from semiconductor technology to laser science.
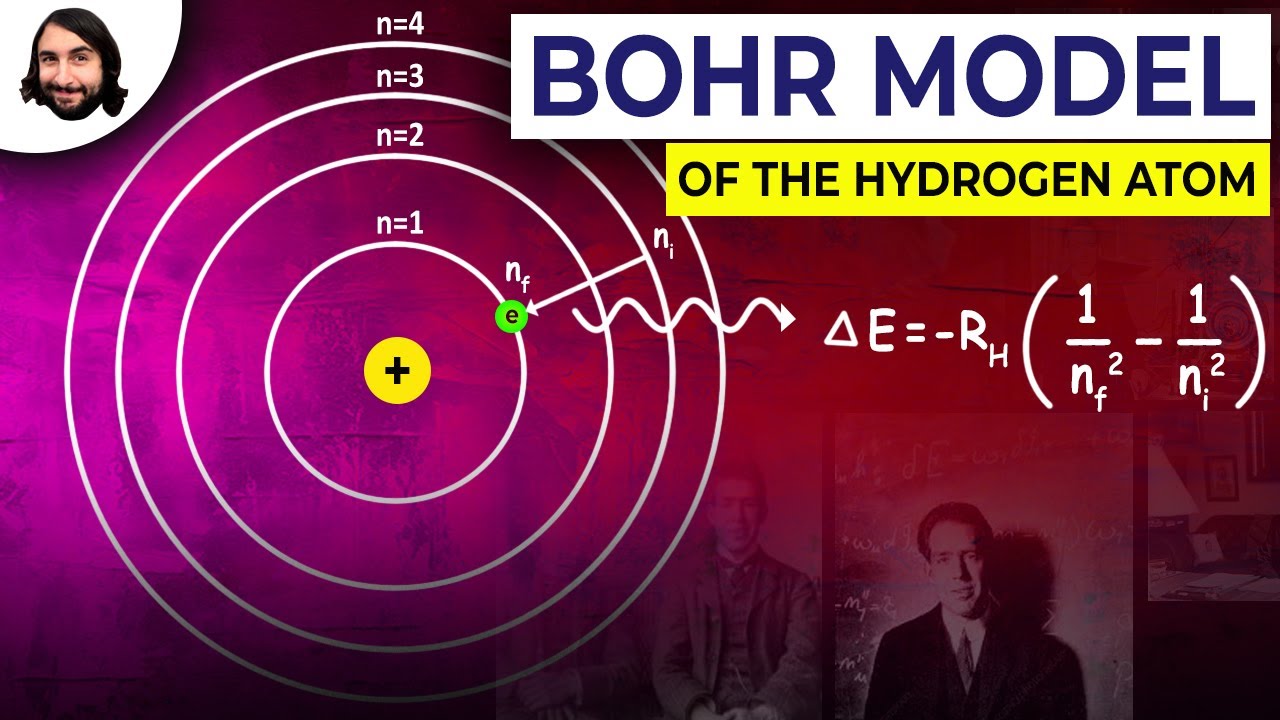
Image: www.youtube.com
Building upon the Foundation: Modern Perspectives on Atomic Structure
The Bohr model, though simplistic, served as an invaluable stepping stone in our journey to understand the atom. It provided a framework that could be expanded upon and refined through further research and innovation. In the years that followed, scientists developed more sophisticated theories, such as quantum mechanics and the shell model, that provided more accurate and comprehensive descriptions of atomic structure and behavior.
These modern models incorporate concepts like electron orbitals, which are probability distributions representing the electron’s position around the nucleus. They also account for the interactions between multiple electrons in atoms, providing a more nuanced understanding of chemical bonding and the properties of different elements.
Embracing the Wonder of the Atom: Unveiling Its Secrets
The journey to understand the atom has been a remarkable odyssey, filled with dazzling discoveries and intellectual breakthroughs. The Bohr model, with its simple but elegant picture of the hydrogen atom, played a pivotal role in this quest. Through digital tools like Gizmos, we can now explore the principles of this model in a vibrant and interactive way, allowing us to appreciate its historical significance and its enduring impact on our understanding of the world around us.
The Bohr model, while not the definitive theory of atomic structure, remains an important stepping stone in our journey to unravel the mysteries of the atom. It serves as a testament to the power of scientific inquiry, reminding us that even the smallest components of the universe can hold immense complexity and beauty.
Bohr Model Of Hydrogen Gizmo Answers
Continuing the Exploration: Beyond the Bohr Model
The Bohr model provides a foundational understanding of atomic structure, but the story doesn’t end there. The world of quantum mechanics, the theory that superseded the Bohr model, offers even deeper insights into the atom’s behavior.
If you’re eager to delve further, consider exploring resources on quantum mechanics, electron orbitals, and the shell model. These advanced concepts offer a more complete picture of the atom, revealing its intricate workings and its crucial role in shaping the universe.
Remember, the journey of scientific discovery is an ongoing process. Each new understanding builds upon the foundation laid by previous generations of scientists, expanding our perspectives and leading us toward a more complete picture of the natural world.






