Have you ever wondered how the precise blend of ingredients in a cake batter determines its texture and overall appeal? Or how the composition of a metal alloy dictates its strength and resilience? Behind these seemingly mundane questions lies a complex world of material science, where a fundamental concept known as the eutectic phase diagram plays a pivotal role. Understanding eutectic systems helps us predict and control the properties of materials, ultimately enhancing their performance in various applications.
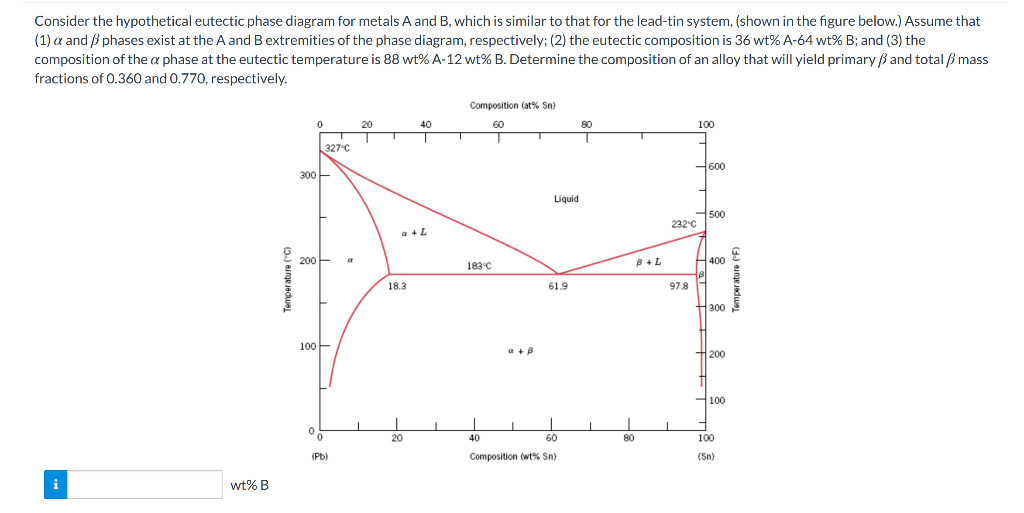
Image: www.chegg.com
At its core, a eutectic phase diagram is a graphical representation of the equilibrium relationships between different phases of a material system, primarily focusing on the melting and solidification behavior of alloys. The term “eutectic” derives from the Greek words “eu” and “tektos,” meaning “well” and “melting,” respectively. It refers to a specific point on the phase diagram where a liquid mixture solidifies directly into a solid mixture of two or more distinct phases, exhibiting unique characteristics compared to the individual components.
Unveiling the Eutectic System: A Deeper Dive
A Pictorial Representation of Material Transformation
Imagine a simple system consisting of two metals, A and B, that can form an alloy. At high temperatures, both metals exist in a liquid state, completely miscible, meaning they can dissolve in each other to form a homogeneous melt. As the temperature decreases, the liquid mixture starts to cool down, eventually reaching a point where the first solid phase begins to form. This point is marked as the “liquidus line” on the phase diagram.
The composition of the solid phase that emerges might differ from the original liquid composition. Consequently, the remaining liquid phase becomes enriched in the component that remains in the liquid state. This process continues until the temperature drops further, reaching the “solidus line” where complete solidification occurs.
Understanding the Eutectic Point
Now, let’s consider the eutectic point. This special point represents the specific composition of the alloy where the liquid phase transforms directly into a mixture of two distinct solid phases upon cooling. This transformation happens at a fixed temperature, known as the eutectic temperature, which is the lowest possible melting point for the alloy system.
In simpler terms, the eutectic point signifies the “perfect blend” where both metals solidify simultaneously at a specific temperature, forming a unique microstructure of intergrown solid phases. This microstructure, often exhibiting a lamellar or layered structure, is directly influenced by the eutectic composition and the cooling rate.
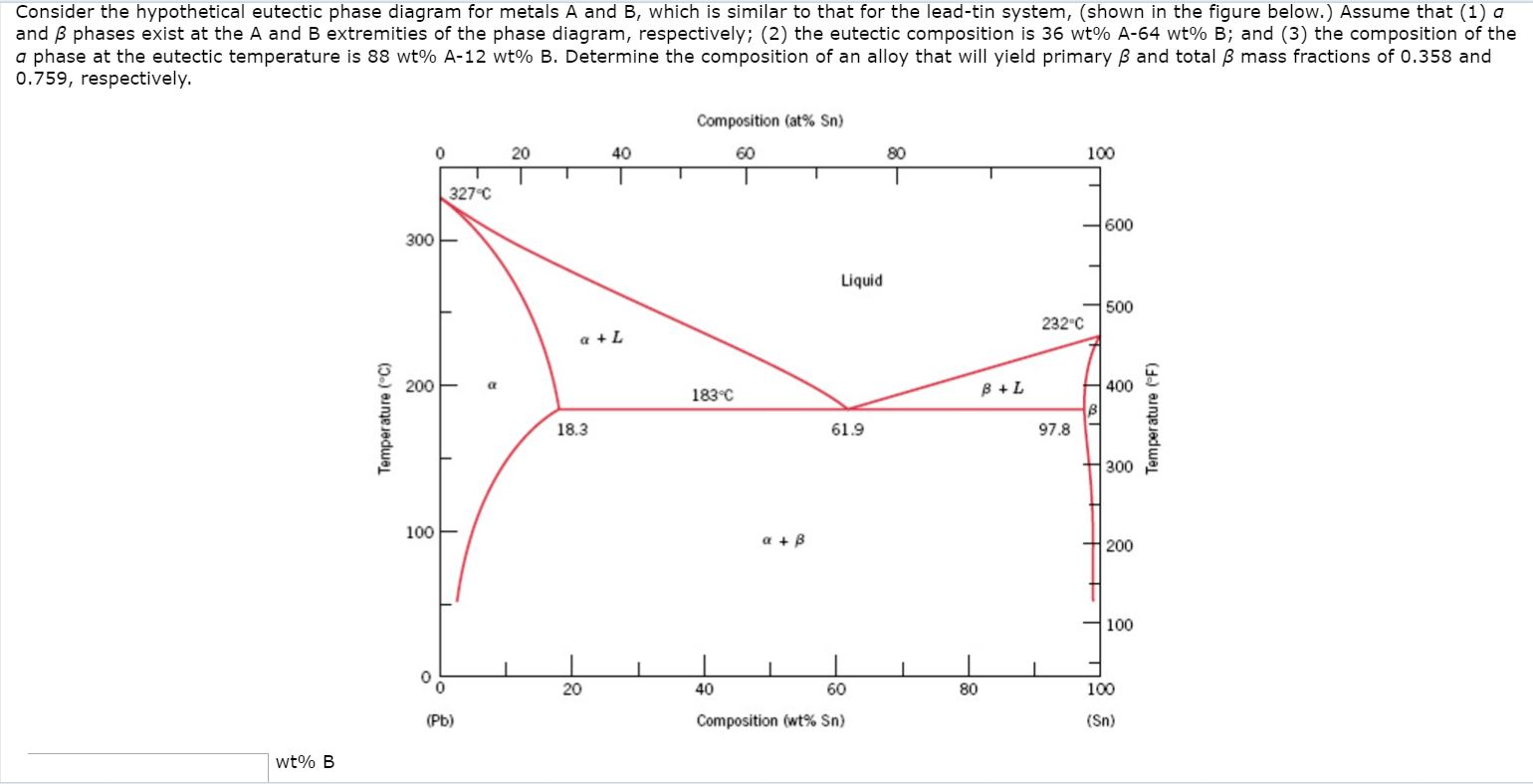
Image: www.chegg.com
Examples of Eutectic Systems
The concept of eutectic systems permeates numerous fields, from metallurgy and materials science to food science and even the study of geological formations.
- Lead-Tin Solder: A common example is lead-tin solder, where a eutectic alloy with approximately 63% tin and 37% lead exhibits the lowest melting point. This property makes it ideal for soldering, as it allows for low-temperature melting and stable bonding of electronic components.
- Aluminum-Silicon Alloys: In the aluminum-silicon system, the eutectic composition results in a microstructure of aluminum crystals embedded in a silicon matrix. This microstructure enhances the mechanical properties of the alloy, making it suitable for automotive components and other structural applications.
- Salt-Water Mixtures: Even everyday phenomena like the freezing of saltwater solutions demonstrate eutectic behavior. The eutectic point of salt and water occurs at approximately -21.1°C. This explains why salt is used to de-ice roads, as the presence of salt lowers the freezing temperature of water, preventing the formation of ice.
Implications of Eutectic Systems in Materials Science
Predicting and Controlling Microstructure and Properties
The eutectic phase diagram serves as a powerful tool in materials science. By understanding the equilibrium relationships between phases, researchers and engineers can predict the microstructure and resulting properties of alloys depending on their composition and processing conditions. For instance, understanding the eutectic point allows the design and optimization of alloys for specific applications, leveraging the unique microstructures that form at the eutectic composition.
Impact on Material Performance
The properties of a eutectic alloy, such as strength, ductility, hardness, and electrical conductivity, can vary significantly depending on its composition and the resulting microstructure. Eutectic systems often exhibit enhanced properties compared to their individual constituent phases. For example, some eutectic alloys display increased strength and hardness due to the formation of fine, intergrown phases, improving their resistance to wear and tear.
Tailoring Alloys for Specific Applications
The ability to manipulate eutectic systems paves the way for designing alloys tailored to specific requirements. By controlling the cooling rate during solidification, the microstructure can be further refined, leading to targeted modifications in the alloy’s properties. This is especially relevant in the fabrication of advanced structural materials, aerospace components, and high-performance electronics.
Emerging Trends in Eutectic Systems Research
Research on eutectic systems is constantly evolving, driven by the pursuit of materials with improved performance and novel functionalities.
Exploring New Eutectic Systems
Researchers are actively investigating new binary and ternary eutectic systems. These explorations encompass broader ranges of materials, including metals, ceramics, and polymers, seeking to unlock novel combinations and properties. The development of high-entropy alloys, utilizing multiple elements in near-equimolar proportions, presents an exciting frontier in eutectic system research. These alloys exhibit intriguing properties, such as exceptional strength, corrosion resistance, and high-temperature stability, opening up new avenues for applications in demanding environments.
Nano-scale Control of Eutectic Microstructures
Advances in materials processing techniques, particularly those involving nano-scale manipulation, are enabling precise control over eutectic microstructures. This allows the creation of materials with tailored properties by precisely manipulating the size, shape, and distribution of the constituent phases. For example, nano-scale eutectic systems can offer improved mechanical properties, enhanced thermal conductivity, and unique electrical characteristics.
Eutectic Systems in Additive Manufacturing
Additive manufacturing, also known as 3D printing, is transforming the way we design and fabricate materials. The application of eutectic systems within additive manufacturing processes holds immense potential. This integration allows for the creation of complex geometries and intricate microstructures, unlocking new possibilities in the fabrication of functional materials and devices with customized properties.
Consider The Hypothetical Eutectic Phase Diagram
Conclusion: A Journey into the Future of Materials Science
In conclusion, understanding the hypothetical eutectic phase diagram is crucial in the realm of materials science. It empowers us to unravel the intricate relationship between composition, processing, and properties, ultimately influencing the design and fabrication of materials with desired characteristics for a wide range of applications. As we continue to explore new eutectic systems, delve into nano-scale precision, and integrate these systems with advanced manufacturing techniques, the scope and impact of this fundamental concept will only continue to expand, shaping the future of materials science and its role in solving the world’s most pressing technical challenges.






