Imagine a world without the dazzling brilliance of diamonds, the life-sustaining properties of oxygen, or the conductive marvel of copper. These are just a few examples of the diverse and fascinating elements that make up our universe, each with its own unique set of properties and defining characteristics. Our exploration begins with the first 30 elements on the periodic table, the foundational blocks upon which all other elements are built.

Image: www.pixazsexy.com
From the lightest element, hydrogen, to the versatile zinc, these elements hold the key to understanding the fundamental building blocks of matter. Join us as we take a journey through the first 30 elements, uncovering their stories, their applications, and their crucial role in shaping our world.
The Periodic Table: A Map of the Elements
Arranging the Elements
The periodic table, a beautifully structured chart, arranges the elements according to their atomic number, which represents the number of protons in an atom’s nucleus. As you move from left to right across the table, the atomic number increases, meaning that each element has one more proton than the one before it. The rows, or periods, represent the element’s electron shell configuration, while the columns, or groups, reflect the elements with similar chemical properties.
Elements 1 to 30: A Closer Look
The first 30 elements in the periodic table are a diverse group, encompassing metals like sodium (Na) and iron (Fe), nonmetals like carbon (C) and chlorine (Cl), and metalloids like silicon (Si). Each element’s unique atomic structure dictates its physical and chemical properties, influencing how it interacts with other elements and its role in various applications.
For instance, hydrogen (H), the lightest and most abundant element in the universe, plays a vital role in stars and is a key component of water. Helium (He), the second lightest element, is known for its light weight and inert nature, frequently used in balloons and in scientific research. Carbon (C), the building block of life, forms the backbone of organic molecules and fuels a vast array of chemical reactions essential for life. Nitrogen (N), a crucial component of our atmosphere, is essential for plant growth and is found in many organic compounds.
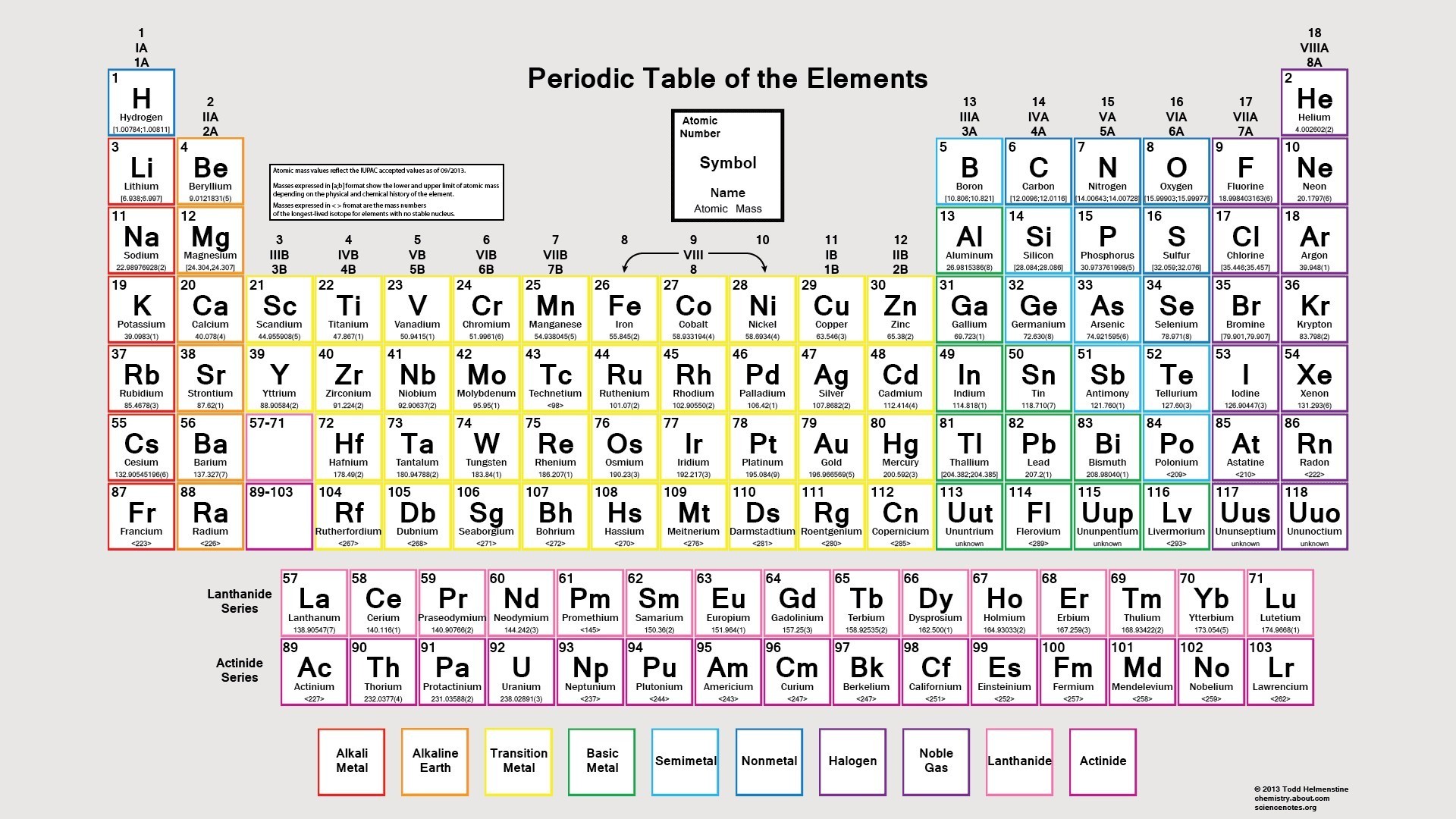
Image: mungfali.com
From Everyday Essentials to Cutting-Edge Technology
The applications of these elements span a wide spectrum, from everyday essentials to cutting-edge technologies. Oxygen (O), a vital ingredient for respiration, is also used in steelmaking and medical applications like oxygen therapy. Silicon (Si), a metalloid, plays a crucial role in the electronics industry, being a key component of semiconductors and solar cells. Copper (Cu), a highly conductive metal, is indispensable for electrical wiring, plumbing, and numerous industrial applications.
Iron (Fe), a strong and durable metal, is a cornerstone of the construction and automotive industries. Sodium (Na), used in table salt and in numerous industrial processes, is also essential for human health. Chlorine (Cl), a reactive nonmetal, plays a vital role in disinfecting water and producing chemical compounds.
Trends and Developments
The field of element research continues to evolve, with advancements in techniques for synthesizing new elements and understanding their properties. The discovery of new isotopes, variations of elements with different neutron numbers, is constantly expanding our knowledge of the elements. Furthermore, the development of new materials and technologies based on elements like graphene and silicon carbide is revolutionizing various industries, from electronics to energy production.
Tips for Exploring the Elements
Embrace the Visuals
The periodic table is your guide to understanding the elements. Familiarize yourself with its structure, the location of different groups and periods, and the color coding used to differentiate between metals, nonmetals, and metalloids.
Explore Element-Specific Resources
For deeper insights into individual elements, explore online resources, books, and documentaries that provide detailed information about their physical properties, chemical behaviors, and applications. Many interactive websites and educational platforms offer engaging ways to learn about the elements.
Frequently Asked Questions
1. What are the most common elements in the universe?
The most abundant elements in the universe are hydrogen (H) and helium (He), accounting for roughly 99.9% of all matter.
2. What is the smallest element?
Hydrogen (H) is the smallest element with only one proton and one electron.
3. What is the heaviest element?
Oganesson (Og) is the heaviest element discovered so far, with an atomic number of 118.
Elements Of Atomic Number 1 To 30
https://youtube.com/watch?v=lajJ1tQOEwY
Conclusion
From the fundamental building blocks of life to the cutting-edge technologies shaping our future, the elements play a vital role in our world. Understanding the first 30 elements on the periodic table provides a foundational knowledge base for exploring the fascinating world of chemistry and its applications. Do you find the realm of elements intriguing? Let us know your thoughts and continue to delve into this captivating world of scientific wonders.






