Imagine a world without the vibrant colors of a sunset, the comforting warmth of a campfire, or the power to light up a room with a flick of a switch. These seemingly simple occurrences are made possible by the extraordinary dance of atoms, the fundamental building blocks of our universe. At the heart of this dance lies the periodic table, a magnificent map that charts the elements that make up everything around us.
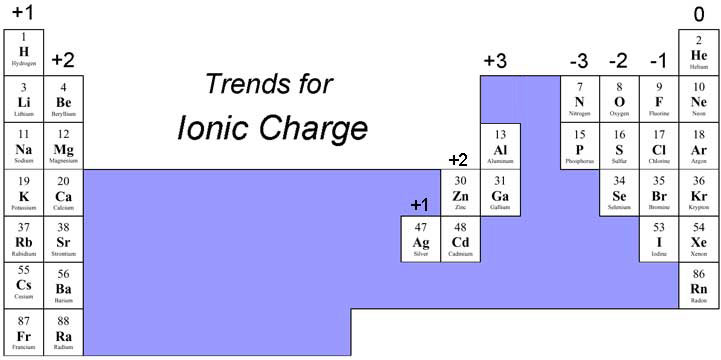
Image: www.chem.fsu.edu
But the table holds a secret, a hidden code that dictates how elements interact with each other, giving rise to an astounding array of properties and reactions. This code is woven into the very fabric of the elements: their charges. Understanding these charges unlocks a deeper appreciation for the intricate world around us, allowing us to comprehend the forces that shape our planet, our bodies, and even the vast expanse of the cosmos.
Delving Deeper into the Periodic Table of Elements and Their Charges
The periodic table, a masterpiece of scientific organization, arranges elements based on their atomic number, the number of protons in their nucleus. Each element, like a unique fingerprint, possesses a distinct arrangement of electrons, negatively charged particles that orbit the nucleus.
The Dance of Charges
The charges associated with elements stem from the delicate balance between protons, positively charged particles found in the nucleus, and electrons. In a neutral atom, the number of protons and electrons is equal, resulting in a net charge of zero.
However, the magic of the periodic table lies in the fact that atoms can gain or lose electrons. When an atom gains an electron, it becomes negatively charged, forming an anion. Conversely, when an atom loses an electron, it acquires a positive charge, transforming into a cation.
Predicting the Charge: The Power of the Periodic Table
The periodic table isn’t just a static chart; it’s a dynamic guide that reveals predictable patterns in the behavior of elements. Understanding these patterns allows us to anticipate the charges elements are likely to acquire.
- Group 1 (Alkali Metals): These elements have a single electron in their outermost shell, eager to give it up to achieve a stable configuration, resulting in a +1 charge.
- Group 2 (Alkaline Earth Metals): With two electrons in their outermost shell, these elements prefer to shed both, yielding a +2 charge.
- Group 17 (Halogens): These elements have seven electrons in their outermost shell, seeking one more to complete their shell, forming a -1 charge.
- Group 18 (Noble Gases): These elements possess a full outermost shell, making them incredibly stable and unreactive. They rarely form ions.

Image: yahoodas.weebly.com
The Importance of Charges: A Symphony of Bonds
Charges are not merely abstract concepts; they hold the key to the formation of chemical bonds, the forces that hold atoms together, creating molecules and compounds. The opposite charges attract, drawing together cations and anions, forming ionic bonds. These bonds are responsible for the formation of countless compounds essential to life, from the salt we sprinkle on our food to the calcium in our bones.
Beyond Bonds: A Glimpse into the World Around Us
The charges of elements play a critical role in a vast array of phenomena:
- Electricity: The flow of electrons, driven by differences in charge, powers our devices and lights our lives.
- Solutions: The interaction between ions and water molecules governs the solubility of various substances, contributing to the intricate balance of life.
- Batteries: The flow of ions between electrodes generates the electrical energy that fuels our portable devices.
- Medicine: Understanding the charges of ions is crucial in the development of drugs, ensuring they bind to the intended targets within the body.
Expert Insights and Actionable Tips
Dr. Sarah Mitchell, a renowned chemist from Stanford University, shares her insights: “The periodic table and the charges of elements are the foundation of chemistry. By understanding these concepts, we can unlock a deeper understanding of the world around us. Embrace this knowledge and use it to explore the fascinating world of chemistry!”
Here are some actionable tips to make the most of your knowledge of element charges:
- Visualize the Charge: Imagine elements “wanting” to achieve a stable configuration, just like a magnet seeking its opposite pole.
- Practice: Build your knowledge by predicting the charges of various elements based on their position on the periodic table.
- Explore Resources: Dive into online resources, interactive simulations, and textbooks to solidify your understanding.
Periodic Table Of Elements And Their Charges
Conclusion
Embarking on a journey of understanding the charges of elements is like stepping into a hidden world of scientific wonders. It unlocks the mysteries of the universe, revealing the forces that shape our environment and drive the processes that sustain life. The periodic table, a seemingly simple chart, is much more than just a collection of elements; it’s a dynamic map of fundamental building blocks, a roadmap to the very essence of matter, guiding us through an incredible universe of charged interactions.
So, embrace the world of elements and their charges, and let your curiosity unravel the secrets that lie within. The world of science is waiting to be explored!






