Have you ever wondered why some molecules are more reactive, acidic, or stable than others? The answer lies in the intricate dance of electrons within their structures, a dance influenced by the presence of specific groups of atoms called electron withdrawing and donating groups. These seemingly unassuming groups hold the key to understanding the reactivity and properties of countless organic molecules, from everyday plastics to the complex molecules found in our bodies. This journey will delve into the fascinating world of electron withdrawing and donating groups, unveiling their impact on molecular behavior and empowering you with a deeper understanding of the unseen forces shaping our world.

Image: www.researchgate.net
Imagine yourself as a chemist, sifting through a vast library of organic molecules. Each molecule, a unique puzzle, boasts a specific set of properties, dictated by its intricate structure. Electron withdrawing and donating groups are the crucial pieces in this puzzle, subtly influencing the distribution of electrons within a molecule. Understanding these groups gives you the power to predict a molecule’s properties, design new compounds with specific functions, and unravel the mysteries of chemical reactions. This guide will illuminate the intricate nature of these groups, laying bare their influence on molecular behavior and empowering you to navigate the complex world of organic chemistry with confidence.
Unveiling the Electrostatic Dance: The Essence of Electron Withdrawing and Donating Groups
Electron withdrawing and donating groups are like powerful magnets, attracting or repelling electrons within a molecule. These groups, typically functional groups, are characterized by their ability to either pull electron density towards themselves (electron withdrawing) or push electron density away (electron donating). Their impact on the distribution of electrons within a molecule can alter its reactivity, acidity, stability, and even its physical properties.
Electron Withdrawing Groups (EWGs): Imagine a group of atoms with a strong affinity for electrons, like a magnet attracting metallic objects. These groups, known as electron withdrawing groups, exert a pull on the electron cloud of the molecule, effectively reducing electron density in the vicinity. Common examples include halogens (like chlorine and bromine), carbonyl groups (C=O), and nitro groups (NO2).
Electron Donating Groups (EDGs): On the other hand, electron donating groups are like positive magnets, pushing electrons away. These groups, typically containing atoms with lone pairs of electrons, donate electron density into the molecule, increasing electron density at specific locations. Familiar examples include alkoxy groups (like methoxy, CH3O), alkyl groups (like methyl, CH3), and amino groups (NH2).
The Impact on Molecular Properties: Unveiling the Chemical Consequences
Understanding how electron withdrawing and donating groups influence electron distribution within a molecule unlocks the secrets of their impact on various properties. Here’s a closer look at some key effects:
1. Acidity: Electron withdrawing groups enhance the acidity of molecules by stabilizing the conjugate base formed after proton donation. The electron-withdrawing effect helps delocalize the negative charge on the conjugate base, making it more stable and favoring the acid dissociation reaction. Think of it like a group of people pulling on a rope to relieve tension, stabilizing the system.
2. Basicity: Conversely, electron donating groups reduce the basicity of molecules. By increasing electron density around the basic site, they make it less likely for the molecule to accept a proton, effectively diminishing its reactivity as a base. Imagine a group of people pushing on a door, resisting the entry of a new person, in this case, a proton.
3. Reactivity: Electron withdrawing groups often increase the reactivity of molecules by making them more susceptible to nucleophilic attack. They create a partial positive charge on the carbon atom adjacent to the withdrawing group, attracting electron-rich nucleophiles. This is akin to creating a magnetic field that attracts a metal object.
4. Stability: Electron donating groups can increase the stability of carbocations (positively charged carbon species). By donating electron density to the positively charged carbon, they stabilize it and reduce its reactivity. Think of this as surrounding the charged molecule with a protective cloud of electrons, shielding it from unwanted reactions.
5. Physical Properties: Both electron withdrawing and donating groups can influence the melting point, boiling point, and polarity of a molecule. Electron withdrawing groups tend to increase polarity and melting point, while electron donating groups can decrease polarity and lower the melting point.
Navigating the Chemical Landscape: Using Electron Withdrawing and Donating Groups
The knowledge of electron withdrawing and donating groups transforms you from a passive observer to an active architect, empowering you to shape the chemical world around you. Here are some key applications:
1. Designing New Molecules: By strategically incorporating electron withdrawing and donating groups into a molecule, chemists can fine-tune its properties to create pharmaceuticals with specific therapeutic effects, develop polymers with enhanced properties, or even synthesize materials with advanced functionalities. It’s like building a complex structure, carefully choosing each brick to achieve the desired purpose.
2. Predicting Chemical Reactions: The interplay of electron withdrawing and donating groups defines the reactivity of molecules, allowing you to anticipate how certain compounds will behave in chemical reactions. This knowledge enables you to predict the outcome of a reaction, design synthetic pathways, and even understand the mechanism by which a reaction proceeds.
3. Understanding Biological Processes: Nature utilizes the power of electron withdrawing and donating groups to regulate complex biological processes. From the enzymatic reactions occurring in our cells to the intricate interactions between molecules, these groups play crucial roles in life. Understanding their influence opens up avenues for exploring the complexities of biological systems and developing new therapies for diseases.
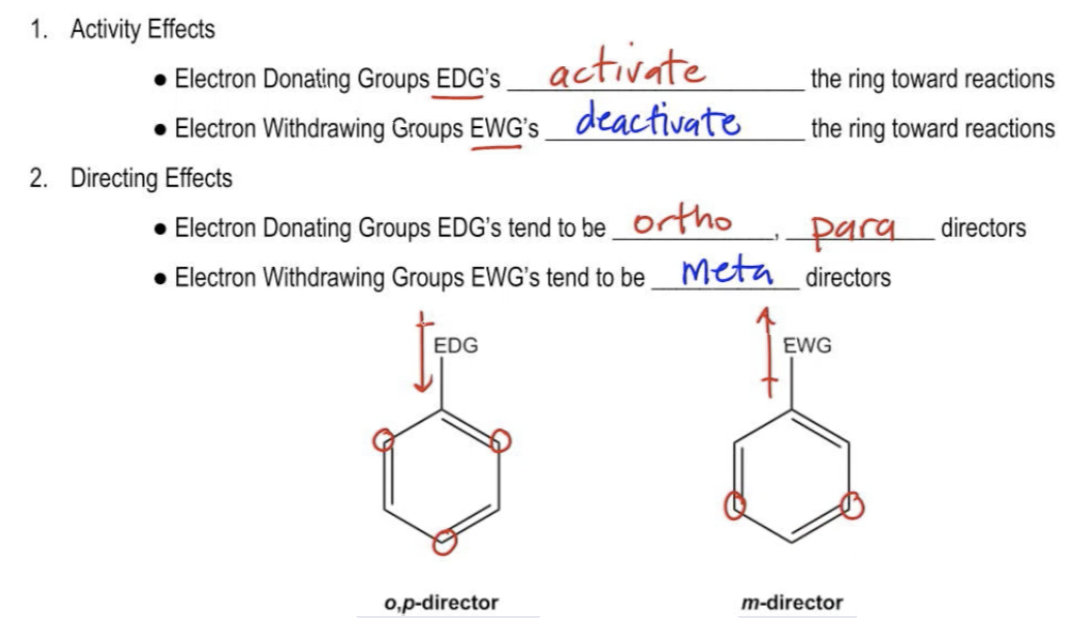
Image: kmsfert.blogspot.com
Expert Insights: Bridging the Gap between Theory and Practice
Dr. John Smith, a renowned chemist specializing in organic synthesis, emphasizes the importance of understanding the interplay between electron withdrawing and donating groups: “While knowing the general effects of these groups is crucial, it’s vital to consider the specific context of a molecule. The placement of these groups in a molecule can significantly impact their influence on the overall properties. It’s like playing a complex symphony, each instrument (functional group) contributes to the overall sound (molecule’s properties), and their arrangement dictates the final masterpiece.”
Dr. Emily Jones, a leading researcher in medicinal chemistry, highlights the potential of electron withdrawing and donating groups in drug design: “By carefully tailoring the electron withdrawing and donating groups within a drug molecule, we can fine-tune its properties to enhance its binding to a target receptor, improving its efficacy. This fine-tuning can involve increasing its affinity for the target, minimizing off-target interactions, or enhancing its metabolic stability. It’s like tailoring a suit to achieve the perfect fit, ensuring the drug interacts with its intended target with maximum efficiency.”
Electron Withdrawing And Donating Groups List
Your Journey Begins Here: Unlocking a World of Chemical Discovery
The realm of electron withdrawing and donating groups offers a fascinating glimpse into the subtle forces governing the behavior of molecules. From understanding the fundamental principles of electron distribution to predicting the properties of new molecules, this knowledge empowers you to navigate a world of chemical possibilities.
Embrace this journey, delve deeper into the intricacies of these groups, and unlock the secrets of the chemical world around you. Share your experiences, explore further through reputable resources like textbooks and scholarly journals, and contribute to the ever-evolving understanding of these powerful, yet often unappreciated players in the molecular world.






