The periodic table, that organized grid of elements, holds within it a wealth of information about the building blocks of our universe. From the inert gases that form the air we breathe to the reactive metals that power our technology, each element has its own unique story. But beyond individual elements, the periodic table reveals fascinating patterns – trends that connect elements into families and groups based on their recurring properties. These trends, from electronegativity to ionization energy, allow us to predict how elements will behave in chemical reactions, forming the foundation of our understanding of chemistry.
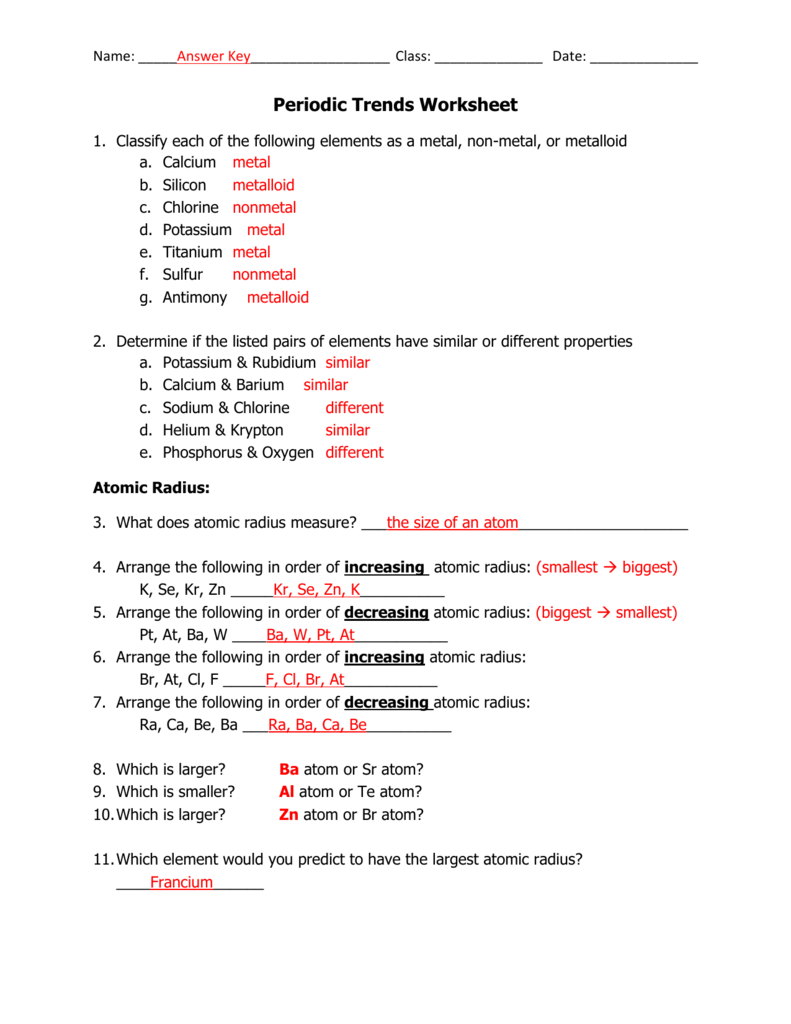
Image: zipworksheet.com
Have you ever wondered how scientists make sense of these intricate relationships? The answer lies in **graphing periodic trends**. By visualizing these patterns, we can gain deeper insights into the behavior of elements and the forces that govern their interactions. This article will explore the world of graphing periodic trends, equipping you with the knowledge and tools to understand this fundamental aspect of chemistry.
Understanding Periodic Trends and Their Representations
The Periodic Table’s Hidden Language
The periodic table, meticulously designed by Dmitri Mendeleev, isn’t just a static list. It’s a dynamic map that reveals the underlying principles of atomic structure. The fundamental properties of elements, like their reactivity, size, and ionization energy, are governed by the arrangement of electrons within their atoms. These properties are not randomly distributed; they follow specific patterns across the table, forming predictable trends.
Visualizing Chemical Behavior
To truly grasp these trends, we need more than just looking at the periodic table. Graphing periodic trends allows us to visualize these patterns and analyze them in depth. By plotting various properties against atomic number, we can observe how values change as we move across a period (horizontal rows) or down a group (vertical columns). This visual representation helps us identify overarching relationships and make predictions about the behavior of elements.

Image: zipworksheet.com
Key Periodic Trends: A Visual Exploration
Ionization Energy: The Energy of Letting Go
Ionization energy refers to the minimum energy required to remove an electron from an atom in its gaseous state. As we move across a period from left to right, ionization energy generally increases. This is because the increasing number of protons in the nucleus attracts the electrons more strongly, making it harder to remove them. On the other hand, moving down a group, ionization energy decreases because the outermost electron is farther from the nucleus, experiencing less attraction and becoming more easily removable.
Electronegativity: The Tug-of-War for Electrons
Electronegativity measures an atom’s tendency to attract electrons in a chemical bond. It follows a similar pattern to ionization energy. Across a period, electronegativity increases due to the stronger nuclear charge pulling electrons closer. Down a group, electronegativity decreases as the outermost electrons are further from the nucleus, experiencing weaker attraction.
Atomic Radius: The Atom’s Size
Atomic radius refers to the average distance between the nucleus and the outermost electron. Moving across a period, atomic radius decreases due to the increasing nuclear charge pulling the electrons closer. However, moving down a group, atomic radius increases because the addition of electron shells pushes the outermost electrons further away from the nucleus.
Electron Affinity: The Attraction of an Electron
Electron affinity is a measure of the change in energy when an electron is added to an atom. While it doesn’t follow the same exact trends as ionization energy and electronegativity, it generally increases across a period and decreases down a group. Elements with a strong affinity for electrons are more likely to form negatively charged ions.
Unlocking the Power of Graphing: Tips and Strategies
The Power of Visual Representation:
Graphing periodic trends goes beyond memorizing facts and figures. It allows us to see the relationships between variables and understand the underlying principles that govern chemical behavior. By visualizing these trends, we can make predictions about the properties of elements and their reactions, deepening our understanding of the vast world of chemistry.
Tips for Effective Graphing:
When graphing periodic trends, there are several tips to ensure accuracy and clarity:
- Choose the right type of graph: The type of graph you choose depends on the data you are presenting. Line graphs are ideal for showing continuous trends across atomic numbers, while bar graphs can be used for comparing values between different elements.
- Label axes correctly: Make sure to label the axes with the correct variables and units. This ensures that the graph is clear and easy to interpret.
- Use a consistent scale: Using a consistent scale for all graphs of periodic trends will allow for accurate comparison between different properties.
- Include a title: A descriptive title is essential for explaining the purpose of the graph and conveying the data being presented.
Expert Advice:
Always strive to connect the patterns you observe in your graphs to the underlying atomic structure. Consider factors like the number of protons, the arrangement of electrons, and the shielding effect of inner electrons. This will deepen your understanding of the trends and enhance your ability to predict chemical behavior.
Frequently Asked Questions:
Q: What are the benefits of graphing periodic trends?
A: Graphing periodic trends helps us visualize and analyze patterns in chemical behavior. It allows us to see relationships between properties and predict how elements will react. This understanding is valuable in various fields, from chemistry research to materials science.
Q: What are some common applications of periodic trends?
A: Periodic trends have diverse applications:
- Predicting chemical reactions: Knowing trends helps predict reactivity and the likelihood of forming compounds.
- Understanding bonding: Trends reveal how elements form bonds and the strengths of those bonds.
- Designing materials: Tailoring material properties by understanding how elemental properties influence them.
Q: Where can I find resources to practice graphing periodic trends?
A: You can find numerous online resources, chemistry textbooks, and educational websites with practice problems, examples, and explanations on graphing periodic trends.
Graphing Periodic Trends Answer Key Pdf
Summarizing the Patterns of the Periodic Table
The periodic table is more than just a list of elements; it is a testament to the organized beauty of the universe. Understanding periodic trends is key to unlocking the secrets of chemistry. By graphing these trends, we visually represent how elements behave, react, and bond. This knowledge empowers you to make informed predictions, understand the underlying principles of atomic structure, and contribute to the ongoing exploration of the chemical world.
Are you fascinated by the periodic trends and their implications? Would you like to delve deeper into specific trends or explore real-world applications of this knowledge? Share your thoughts and questions in the comments below. Let’s continue this journey of discovery together.






